Lab one slide share for chm 2
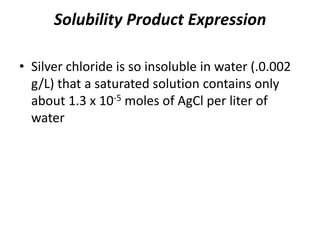
- 1. Solubility Product Expression • Silver chloride is so insoluble in water (.0.002 g/L) that a saturated solution contains only about 1.3 x 10-5 moles of AgCl per liter of water
- 2. 1.3 x 10-5 moles of AgCl • AgCl(s) <-> Ag+(aq) + Cl-(aq) • Ag+ = 107. amu • Cl- = 35.45 amu== 143 amu • 143.34 g/mol
- 3. AgCl(s) <-> Ag+(aq) + Cl-(aq)
- 4. • (Water isn't included in the equilibrium constant expression because it is neither consumed nor produced in this reaction, even though it is a vital component of the system.)
- 5. • The [Ag+] and [Cl-] terms represent the concentrations of the Ag+ and Cl- ions in moles per liter when this solution is at equilibrium
- 6. • The third term [AgCl] is more ambiguous. It doesn't represent the concentration of AgCl dissolved in water because we assume that AgCl dissociates into Ag+ ions and Cl- ions when it dissolves in water.
- 7. • It can't represent the amount of solid AgCl in the system because the equilibrium is not affected by the amount of excess solid added to the system.
- 8. • The [AgCl] term has to be translated quite literally as the number of moles of AgCl in a liter of solid AgCl.
- 9. • The concentration of solid AgCl can be calculated from its density and the molar mass of AgCl.
- 11. • This quantity is a constant, however. The number of moles per liter in solid AgCl is the same at the start of the reaction as it is when the reaction reaches equilibrium
- 12. • Since the [AgCl] term is a constant, which has no effect on the equilibrium, it is built into the equilibrium constant for the reaction. • [Ag+][Cl-] = Kc x [AgCl]
- 13. [Ag+][Cl-] = Kc x [AgCl] • This equation suggests that the product of the equilibrium concentrations of the Ag+ and Cl- ions in this solution is equal to a constant. • [Ag+][Cl-] = Kc x [AgCl] • [Ag+][Cl-] = Kc x [1] • [Ag+][Cl-] = Kc
- 14. • Since this constant is proportional to the solubility of the salt, it is called the solubility product equilibrium constant for the reaction, or Ksp.
- 15. solubility product equilibrium constant • Ksp = [Ag+][Cl-] = 100 • [50 moles][50 moles-]= 100 get a solid • [25moles][75 moles-]= no solid
- 16. • Ksp = [Ag+][Cl-] • When= 1.3 x 10-5 moles of AgCl is reached
- 17. raised to a power equal • The Ksp expression for a salt is the product of the concentrations of the ions, with each concentration raised to a power equal to the coefficient of that ion in the balanced equation for the solubility equilibrium
- 18. The Relationship Between Ksp And the Solubility of a Salt • Ksp is called the solubility product because it is literally the product of the solubilities of the ions in moles per liter. The solubility product of a salt can therefore be calculated from its solubility, or vice versa.
- 19. AgBr crystals that do not absorb light • Photographic films are based on the sensitivity of AgBr to light. When light hits a crystal of AgBr, a small fraction of the Ag+ ions are reduced to silver metal. The rest of the Ag+ ions in these crystals are reduced to silver metal when the film is developed. AgBr crystals that do not absorb light are then removed from the film to "fix" the image
- 20. solubility of AgBr in water • Example: Let's calculate the solubility of AgBr in water in grams per liter, to see whether AgBr can be removed by simply washing the film.
- 21. AgBr(s) <-> Ag+(aq) + Br-(aq) • When the combined concentrations of the anion and cation reach this-the precipitate will form • Ksp = [Ag+][Br-] = 5.0 x 10-13
- 22. can't be solved for two unknowns • One equation can't be solved for two unknowns the Ag+ and Br- ion concentrations. We can generate a second equation, however, by noting that one Ag+ ion is released for every Br- ion. Because there is no other source of either ion in this solution, the concentrations of these ions at equilibrium must be the same.
- 23. • [Ag+] = [Br-] • Substituting this equation into the Ksp expression gives the following result. • [Ag+]2 = 5.0 x 10-13 • Taking the square root of both sides of this equation gives the equilibrium concentrations of the Ag+ and Br- ions.
- 24. . • [Ag+] = [Br-] = 7.1 x 10-7M • Once we know how many moles of AgBr dissolve in a liter of water, we can calculate the solubility in grams per liter.
- 25. . • The solubility of AgBr in water is only 0.00013 gram per liter. It therefore isn't practical to try to wash the unexposed AgBr off photographic film with water.
- 26. • Solubility product calculations with 1:1 salts such as AgBr are relatively easy to perform. In order to extend such calculations to compounds with more complex formulas we need to understand the relationship between the solubility of a salt and the concentrations of its ions at equilibrium.
- 27. Confirmation of Silver • (Ag+) • Save 5 ml of this solution immediately-just for safe keeping
- 28. I need the unknown number from Samantha and Tyler • Save 5 ml of this solution immediately-just for safe keeping • This number should match your locker • Silver , mercury and lead • Drop as chlorides • Heat separate the lead
- 29. • Silver forms a soluble complex ion with aqueous ammonia. The presence of silver is confirmed by dissolving any remaining solid residue in 6 M NH3 (aq) and then re- precipitating the chloride by freeing the silver ion from the complex ion using 6 M acid
- 30. • Add ~5 drops of 6 M NH3 (aq) to the solid from A, keeping your test tube near the inlet of the fume exhaust vent. The solid should dissolve, but if any precipitate remains, centrifuge and proceed using only the centrifugate
- 31. • . Add 6 M HNO3 to the solution until the solution is acidified, using pH indicator paper to test for acidification. A white precipitate (AgCl) confirms the presence of Ag+. The Cl- needed for precipitation will be present from the prior dissolution of AgCl
- 32. • Lead (Pb2+) Lead chloride is almost three times more soluble in hot water than cold. One may use this as a basis for separating it from silver chloride. The presence of lead is then confirmed by precipitation of yellow lead chromate.
- 33. confirmed by precipitation of yellow lead chromate.
