Classifying matter
- 3. • Matter is anything that has mass and occupies space. • Matter can be invisible. – Air is matter, but it cannot be seen. • Matter appears to be continuous and unbroken. – Matter is actually discontinuous. It is made up of tiny particles call atoms. 3
- 4. An apparently empty test tube is submerged, mouth downward in water. Only a small volume of water rises into the tube, which is actually filled with invisible matter–air. 4 3.1
- 6. 6
- 7. 7
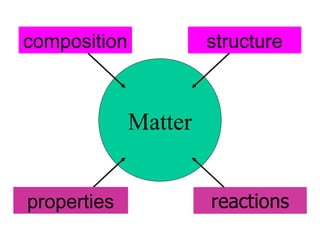
- 9. Matter refers to all of the materials that make up the universe. 9
- 10. Classification of matter: A pure substance is always homogeneous in composition, whereas a mixture always contains two or more substances and may be either homogeneous or heterogeneous. 3.2 10
- 11. Pure Substance A particular kind of matter that has a fixed composition and distinct properties. Examples ammonia, water, and oxygen. 11
- 12. Elements 12
- 13. An element is a fundamental or elementary substance that cannot be broken down into simpler substances by any means 13
- 14. • All known substances on Earth and probably the universe are formed by combinations of more than 100 elements. • Each element has a number. – Beginning with hydrogen as 1 the elements are numbered in order of increasing complexity. 14
- 15. • Most substances can be decomposed into two or more simpler substances. – Water can be decomposed into hydrogen and oxygen. – Table salt can be decomposed into sodium and chlorine. • An element cannot be decomposed into a simpler substance. 15
- 17. • Elements are not distributed equally by nature. – Oxygen is the most abundant element in the human body (65%). – Oxygen is the most abundant element in the crust of the earth (49.2%).. • In the universe the most abundant element is hydrogen (91%) and the second most abundant element is helium (8.75%). 17
- 20. Sources of Element Names GreekColor • Iodine: from the Greek iodes meaning violet. • Fluorine: from the Latin fluere meaning to LatinProperty flow. German- • Bismuth: from the German Color weisse mass which means white mass. Location • Germanium: discovered in 1866 by a German chemist. Famous- • Einsteinium: named for Albert Einstein. 20 Scientists
- 21. Compounds 21
- 22. A compound is a distinct substance that contains two or more elements combined in a definite proportion by weight. Compounds can be decomposed chemically into simpler substances–that is, into simpler compounds or elements. 22
- 23. There are two types of compounds: molecular and ionic. 23
- 24. Molecules 24
- 25. A molecule is the smallest uncharged individual unit of a compound formed by the union of two or more atoms. 25
- 26. • A water molecule consists of two hydrogen atoms and one oxygen atom. • If it is subdivided the water molecule will be destroyed and hydrogen and oxygen will be formed. 26 3.5
- 28. An ion is a positively or negatively charged atom or group of atoms. 28
- 29. A cation is a positively charged ion. 29 3.5
- 30. An anion is a negatively charged ion. 30 3.5
- 31. Ionic compounds are held together by attractive forces between positively and negatively charged ions. 31
- 32. Sodium Chloride The ultimate particles of sodium chloride are positively charged sodium ions and negatively charged chloride ions. 32 3.5
- 33. Compounds can be classified as molecular or ionic. Ionic compounds are held together by attractive forces between their positive and negative charges. Molecular compounds are held together by covalent bonds. 33 3.4
- 34. Mixture Matter containing 2 or more substances that are present in variable amounts. Mixtures are variable in composition. They can be homogeneous or heterogeneous. 34
- 35. Homogeneous Mixture (Solution) A homogeneous mixture of 2 or more substances. It has one phase. Example Sugar and water. Before the sugar and water are mixed each is a separate phase. After mixing the sugar is evenly dispersed throughout the volume of the water. 35
- 36. Heterogeneous Mixture A heterogeneous mixture consists of 2 or more phases. Example Sugar and fine white sand. The amount of sugar relative to sand can be varied. The sugar and sand each retain their own properties. 36
- 37. Heterogeneous Mixture A heterogeneous mixture consists of 2 or more phases. Example • Iron (II) sulfide (FeS) is 63.5% Fe and 36.5% S by mass. • Mixing iron and sulfur in these proportions does not form iron (II) sulfide. Two phases are present: a sulfur phase and an iron phase. • If the mixture is heated strongly a chemical reaction occurs and iron (II) sulfide is formed. • FeS is a compound of iron and sulfur and has 37 none of the properties of iron and sulfur.
- 38. Mixture of iron and sulfur Compound of iron and sulfur Formula Has no definite formula: consists of Fe and S. FeS Composition Contains Fe and S in any proportion by mass. 63.5% Fe and 36.5% S by mass. Separation Fe and S can be separated by physical means. Fe and S can be separated only by chemical change. 38
- 39. 39
- 41. • A symbol stands for – the element itself – one atom of the element – a particular quantity of the element 41
- 42. Rules governing symbols of the elements are: 1. Symbols have either one or two letters. 2. If one letter is used it is capitalized. C hydrogen H carbon 3. If two letters are used, only Ne barium Ba neon the first is capitalized. 42
- 43. These symbols have carried over from the earlier names of the A number of symbols Latin). to the sameconnection with the element. elements (usually start with have no letter as the element. Most symbols appear 43
- 44. 44
- 46. Metals 46
- 48. • Metals are solid at room temperature. – Mercury is an exception. At room temperature it is a liquid. • Metals are good conductors of heat and electricity. Most elements are metals physical properties of or • Metals are malleable (they can be rolledmetals hammered into sheets). • Metals have high luster (they are shiny). 48
- 49. • Metals are ductile (they can be drawn into wires). • Most metals have a high melting point. Most elements are metals • Metals have high densities 49
- 51. Chemical Properties of Metals • Metals have little tendency to combine with each other to form compounds. • Many metals readily combine with nonmetals to form ionic compounds. – They can combine with sulfur. oxygen. chlorine. – In nature, minerals are formed by combinations of the more reactive metals combined with other elements. 51
- 52. Chemical Properties of Metals – A few of the less reactive metals such as copper, silver and gold are found in the free state. – Metals can mix with each other to form alloys. Brass is a mixture of copper and zinc. Bronze is a mixture of copper and tin. Steel is a mixture of carbon and iron. 52
- 53. Nonmetals 53
- 54. Physical Properties of Nonmetals • Lack luster (they are dull) • Have relatively low melting points • Have low densities. • Poor conductors of heat and electricity • At room temperature carbon, phosphorous, sulfur, selenium, and iodine are solids. 54
- 55. Physical State at Room Temperature phosphorous carbon Solid selenium sulfur iodine 55
- 56. Physical State at Room Temperature liquid bromine 56
- 57. Physical State at Room Temperature nitrogen, oxygen gas fluorine, chlorine helium, neon, argon, krypton, xenon, radon 57
- 58. Metalloids 58
- 59. Metalloids have properties that are intermediate between metals and nonmetals 59
- 60. The Metalloids 1. boron 2. silicon 3. germanium 4. arsenic 5. antimony 6. tellurium 7. polonium 60
- 61. Nonmetals found to to left of the the metalloids. Metals are are found thethe right of metalloids 61
- 62. In 1869 Dimitri Mendeleev of Russia and Lothar Meyer of Germany independently published periodic arrangements of the elements based on increasing atomic masses. Mendeleev’s arrangement is the precursor to the modern periodic table. 62
- 63. Period numbers correspond Horizontal rows are to the highest occupied called periods. energy level. 63 10.14
- 64. Elements in the A groups Elements in the B groups with similar Groups are numbered are designated organized properties are transition are designated with Roman numerals. in groups or families. representative elements. elements. 64 10.14
- 65. Period number corresponds with the highest energy level occupied by electrons in that period. 65 10.17
- 66. The group numbersfamily have the same The elements of a for the representative outermost electron configurationnumber that elements are equal to the total except of outermost electrons in the atoms of the group. the electrons are in different energy levels. 66 10.17
- 67. Noble Gas Halogen Group Alkali Metal Alkali Earth Metal Period
- 68. Periodic Trends in Atomic Properties 68
- 69. Characteristic properties and trends of the elements are the basis of the periodic table’s design. 69
- 70. These trends allow us to use the periodic table to accurately predict properties and reactions of a wide variety of substances. 70
- 71. Atomic Radius 71
- 72. Radii of atoms increase down a group. For each step down a group, electrons enter the next higher energy level. 72 11.2
- 73. Radii of atoms tend to decrease from left to right across a period. This Each time an For increase in positive is added electron nuclear representative a charge pulls all proton is within elements a added to electrons closer the same period to nucleus. the energy level nucleus. remains constant as electrons are added. 73 11.2
- 74. Radii of atoms tend to decrease from left to right across a period. Each This time an For increase in electron is added positive nuclear representative a proton pulls all charge is within elements added to electrons closer the same period to nucleus. the energy level nucleus. remains constant as electrons are added. 74 11.2
- 76. The ionization energy of an atom is the energy required to remove an electron from an atom. Na + ionization energy → Na+ + e- 76
- 77. As each succeeding electron is removed from an atom ever higher energies are required. 77
- 78. The Atom Atom is the basic unit of an element, made up of even smaller particles called subatomic particles. There are three fundamental components (subatomic particles) that are important in chemistry: Electron, Proton and Neutron. The protons and neutrons of an atom are packed in an extremely small nucleus. Electrons are shown as ‘clouds’ around the nucleus.
- 79. The Structure of the Atom The Structure of the Atom Electron (cloud) Nucleus Figure above shows the location of the protons, Neutrons and electrons in an atom
- 80. Subatomic Particles mass p = mass n = 1840 x mass e-
- 81. Atomic Numbers of the Elements 81
- 82. Atomic Number, Mass Number & Isotopes Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + number of neutrons Isotopes are atoms of the same element (X) with different numbers of neutrons in their nuclei Mass Number Atomic Number A ZX Element Symbol
- 83. • The atomic number of an element is equal to the number of protons in the nucleus of that element. • The atomic number of an atom determines which element the atom is. 83
- 84. Every atom with an atomic number of 1 is a hydrogen atom. Every hydrogen atom contains 1 proton in its nucleus. 84
- 85. Every atom with an atomic number of 6 is a carbon atom. Every carbon atom contains 6 protons in its nucleus. 85
- 86. atomic number Every atom with an atomic number of 1 is a hydrogen atom. 1 proton in the nucleus H 1 86
- 87. atomic number Every atom with an atomic number of 6 is a carbon atom. 6 protons in the nucleus C 6 87
- 88. atomic number U 92 Every atom with an atomic number of 92 is a uranium atom. 92 protons in the nucleus 88
- 90. • Atoms of the same element can have different masses. • They always have the same number of protons, but they can have different numbers of neutrons in their nuclei. • The difference in the number of neutrons accounts for the difference in mass. • These are isotopes of the same element. 90
- 92. Isotopic Notation 6 protons + 6 neutrons 12 C 6 6 protons 92
- 93. Isotopic Notation 6 protons + 8 neutrons 14 C 6 6 protons 93
- 94. Isotopic Notation 8 protons + 8 neutrons 16 O 8 8 protons 94
- 95. Isotopic Notation 8 protons + 9 neutrons 17 O 8 8 protons 95
- 96. Isotopic Notation 8 protons + 10 neutrons 18 O 8 8 protons 96
- 97. Hydrogen has three isotopes 1 proton 1 proton 1 proton 0 neutrons 1 neutron 2 neutrons 97
- 98. Relationship Between Mass Number and Atomic Number 98
- 99. The mass number minus the atomic number equals the number of neutrons in the nucleus. mass number atomic number atomic mass number number 109 47 = = number of neutrons 62 99
- 101. As n increases, the energy of the electron increases. The first four principal energy levels of an atom. Each level is assigned a principal quantum number n. 101 10.7
- 102. 10.7, 10.8 Each principal energy level is subdivided into sublevels. 102
- 103. Within sublevels the electrons are found in orbitals. An s orbital is spherical in shape. The spherical surface encloses a space where there is a 90% probability that the electron may be found. 103 10.10
- 104. An atomic orbital can hold a maximum of two electrons. An electron can spin in one of two possible directions represented by ↑ or ↓. The two electrons that occupy an atomic orbital must have opposite spins. This is known as the Pauli Exclusion Principal. 104 10.10
- 105. A p sublevel is made up of three orbitals. Each p orbital has two lobes. Each p orbital can hold a maximum of two electrons. A p sublevel can hold a maximum of 6 electrons. 10.10 105
- 106. pz The three p orbitals share a common center. py px The three p orbitals point in different directions. 106 10.10
- 107. A d sublevel is made up of five orbitals. The five d orbitals all point in different directions. Each d orbital can hold a maximum of two electrons. A d sublevel can hold a maximum of 10 electrons. 10.11 107
- 108. 10.8 10.10 10.11 Number of Orbitals in a Sublevel 108
- 109. Distribution of Subshells by Principal Energy Level n=1 1s n=2 2s 2p 2p 2p n = 3 3s 3p 3p 3p 3d 3d 3d 3d 3d n = 4 4s 4p 4p 4p 4d 4d 4d 4d 4d 4f 4f 4f 4f 4f 4f 4f 109
- 110. Nuclear makeup and electronic structure of each principal energy level of an atom. number of protons and electrons number of neutrons in thein each sublevel nucleus 110 10.13
- 111. Electron Configuration Number of electrons in sublevel orbitals Arrangement of electrons within their respective sublevels. 2p 6 Principal Type of orbital energy level 111
- 112. The electron configuration of any of the noble gas elements can be represented by the symbol of the element enclosed in square brackets. B 1s22s22p1 [He]2s22p1 Na 1s22s22p63s1 [Ne]3s1 Cl 1s22s22p63s23p5 [Ne]3s23p5 112
- 113. The electron configuration of argon is Ar 1s22s22p63s23p6 The elements after argon are potassium and calcium. Instead of entering a 3d orbital, the valence electrons of these elements enter the 4s orbital. K 1s22s22p63s23p64s1 [Ar]4s1 Ca 1s22s22p63s23p6 4s2 [Ar]4s2 113
- 114. d orbital numbers are 1 less than d orbital filling the period number 10.16 Arrangement of electrons according to sublevel being filled. 114
- 115. f orbital numbers are 2 less than fthe period number orbital filling 10.16 Arrangement of electrons according to sublevel being filled. 115
