SBP Midyear F5 P3 (soalan)
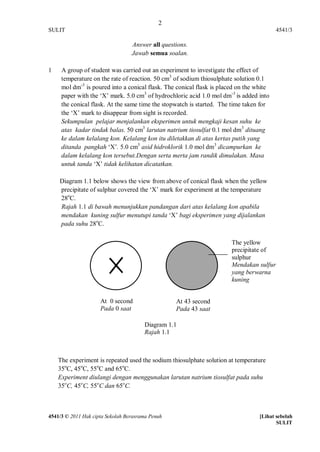
- 1. 2 SULIT 4541/3 Answer all questions. Jawab semua soalan. 1 A group of student was carried out an experiment to investigate the effect of temperature on the rate of reaction. 50 cm3 of sodium thiosulphate solution 0.1 mol dm-3 is poured into a conical flask. The conical flask is placed on the white paper with the ‘X’ mark. 5.0 cm3 of hydrochloric acid 1.0 mol dm-3 is added into the conical flask. At the same time the stopwatch is started. The time taken for the ‘X’ mark to disappear from sight is recorded. Sekumpulan pelajar menjalankan eksperimen untuk mengkaji kesan suhu ke atas kadar tindak balas. 50 cm3 larutan natrium tiosulfat 0.1 mol dm3 dituang ke dalam kelalang kon. Kelalang kon itu diletakkan di atas kertas putih yang ditanda pangkah ‘X’. 5.0 cm3 asid hidroklorik 1.0 mol dm3 dicampurkan ke dalam kelalang kon tersebut.Dengan serta merta jam randik dimulakan. Masa untuk tanda ‘X’ tidak kelihatan dicatatkan. Diagram 1.1 below shows the view from above of conical flask when the yellow precipitate of sulphur covered the ‘X’ mark for experiment at the temperature 28oC. Rajah 1.1 di bawah menunjukkan pandangan dari atas kelalang kon apabila mendakan kuning sulfur menutupi tanda ‘X’ bagi eksperimen yang dijalankan pada suhu 28oC. The yellow precipitate of sulphur Mendakan sulfur yang berwarna kuning At 0 second At 43 second Pada 0 saat Pada 43 saat Diagram 1.1 Masa 0 Rajah 1.1 Masa 0 saat saat Masa 0 saat Masa 0 saat Masa 0 saat The experiment is repeated used the sodium thiosulphate solution at temperature Masa 0 saat 35oC, 45oC, 55oC and 65oC. Experiment diulangi dengan menggunakan larutan natrium tiosulfat pada suhu 35oC, 45oC, 55oC dan 65oC. 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 2. 3 SULIT 4541/3 For Diagram 1.2 shows the readings of the stopwatch for each set at different examiner’s use temperatures. Rajah 1.2 menunjukkan bacaan jam randik bagi setiap tindak balas pada suhu berlainan. At 350C At 450C Pada 350C: ………….. s Pada 450C : ………….. s At 550C At 650C Pada 550C : ………….. s Pada 650C: ………….. s Diagram 1.2 Rajah 1.2 Rajah 1.2 (a) Record the time taken for each reaction in the spaces provided. 1(a) Catatkan masa bagi setiap tindak balas pada ruang yang disediakan. [3 marks] 3 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 3. 4 SULIT 4541/3For examiner’s o o use (b) Construct a table and record the temperature starts from 28 C until 65 C , time 1 and for this experiment. time Bina satu jadual dan rekodkan suhu bermula 28oC sehingga 65oC, masa dan 1 1(b) dalam eksperimen ini. masa [3 marks] 3 1 (c) Plot a graph of temperature against on the graph paper provided on the time page 5. 1 Lukiskan graf suhu melawan pada kertas graf yang disediakan di muka 1(c) masa surat 5. [3 marks] 3 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 4. 5 SULIT 4541/3 Question 1 (c) 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 5. 6 SULIT 4541/3 For examiner’s (d) Based on the graph in (c), state the relationship between the rate of reaction use and temperature. Berdasarkan graf di (c), nyatakan hubungan di antara kadar tindak balas dengan suhu. ………………………………………………………………………………… 1(d) ………………………………………………………………………………… [3 marks] (e) For this experiment state: 3 Bagi eksperimen ini nyatakan: (i) The manipulated variable: Pemboleh ubah di manipulasikan: …………………………………………………………………………. (ii) The responding variable: Pemboleh ubah bergerak balas: ………………………………………………………………………….. (iii) The constant variable: Pemboleh ubah dimalarkan: 1(e) ………………………………………………………………………….. [3 marks] 3 (f) Based on this experiment, state the operational definition for the rate of reaction. Berdasarkan eksperimen ini, nyatakan definisi secara operasi bagi kadar tindak balas. ……………………………………………………………………………………………….. 1(f) ……………………………………………………………………………………………….. [3 marks] 3 Total 1 18 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 6. 7 SULIT 4541/3 2 An experiment was carried out to construct the electrochemical series based the ability of a metal to displace another metal from its salt solution. Satu eksperimen telah dijalankan untuk membina siri elektrokimia berdasarkan kebolehan sesuatu logam menyesarkan logam lain daripada larutan garamnya. Diagram 2.1 shows the set-up of apparatus for the experiment. Rajah 2.1 menunjukkan susunan radas bagi eksperimen tersebut. Test tube At the beginning After 10 minutes Tabung uji Awal Selepas 10 minit Copper(II) Copper(II) sulphate solution sulphate solution (blue colour) (light blue) Larutan Larutan Metal X A Metal X kuprum(II) sulfat kuprum(II) sulfat Logam X (biru) Logam X (biru pudar) Brown solid Pepejal perang Magnesium Magnesium sulphate solution sulphate solution Larutan Larutan B magnesium Metal X magnesium Metal X sulfat Logam X sulfat Logam X Copper(II) Copper(II) sulphate solution sulphate solution (blue colour) (light blue) Larutan Magnesium Larutan Magnesium C kuprum(II) sulfat ribbon kuprum(II) sulfat ribbon (biru) Pita (biru pudar) Pita magnesium magnesium Brown solid Pepejal perang Diagram 2.1 Rajah 2.1 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 7. 8 SULIT 4541/3 For (a) Write the observation for test tube A, B and C in Table 2.1. examiner’s use Tuliskan pemerhatian bagi tabung uji A, B dan C dalam Jadual 2.1. Test tube Observation Tabung uji Pemerhatian A B C Table 2.1 2(a) Jadual 2.1 [ 3 marks] 3 (b) State the inference from the observation in test tube A. Nyatakan inferens berdasarkan pemerhatian dalam tabung uji A. ………………………………………………………………………………… 2(b) ………………………………………………………………………………… [ 3 marks] 3 (c) Based on the observations in the experiment, arrange all the metals in descending order of their electropositivity. Berdasarkan pemerhatian-pemerhatian dalam eksperimen, susunkan semua logam dalam keelektropositifan menurun. 2(c) …………………………………………………………………………..……… [3 marks] 3 (d) If metal X in test tube A is replaced by silver metal, predict the observation for the intensity of blue colour of copper(II) sulphate, CuSO 4 solution. Jika logam X dalam tabung uji A digantikan dengan logam argentum, ramalkan pemerhatian bagi keamatan warna biru larutan kuprum(II) sulfat, 2(d) CuSO4. …………………………………………………………………………..…… 3 [3 marks] 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 8. 9 SULIT 4541/3 For (e) examiner’s Tin, Sn Aluminium, Al use Stanum, Sn Aluminium , Al Lead, Pb Iron, Fe Plumbum, Pb Ferum, Fe Classify the following metals into more electropositive than metal X and less electropositive than metal X. Kelaskan logam-logam berikut kepada lebih elekropositif daripada logam X dan kurang elektropositif daripada logam X. More electropositive Less electropositive Lebih elektropositif Kurang elektropositif 2(e) [3 marks] 3 Total 2 15 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
- 9. 10 SULIT 4541/3 3 Acids only show their acidic properties when they are dissolved in water. Asid hanya menunjukkan sifat keasidannya apabila dilarutkan ke dalam air. Based on the statement, plan a laboratory experiment to investigate the role of water in showing the properties of acid. You are given the glacial ethanoic acid and magnesium ribbon. Berdasarkan pernyataan tersebut, rancang satu eksperimen dalam makmal untuk mengkaji peranan air dalam menunjukkan sifat asid. Anda dibekalkan asid etanoik glasial dan pita magnesium. Your planning must include the following items: Perancangan anda hendaklah mengandungi perkara-perkara berikut: (a) Statement of the problem Pernyataan masalah (b) All the variables Semua pembolehubah (c) Statement of the hypothesis Pernyataan hipotesis (d) List of materials and apparatus Senarai bahan dan radas (e) Procedure of the experiment Prosedur eksperimen (f) Tabulation of data Penjadualan data [ 17 marks ] END OF QUESTION PAPER KERTAS SOALAN TAMAT 4541/3 © 2011 Hak cipta Sekolah Berasrama Penuh [Lihat sebelah SULIT
