Lecture 19.2- pH
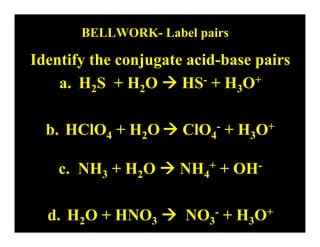
- 1. BELLWORK- Label pairs Identify the conjugate acid-base pairs a. H2S + H2O HS- + H3O+ b. HClO4 + H2O ClO4- + H3O+ c. NH3 + H2O NH4+ + OH- d. H2O + HNO3 NO3- + H3O+
- 2. The pH scale measures the hydrogen ion concentration[H+] of a solution.
- 3. A pH of 7 is neutral A pH less than 7 is acidic (litmus is red) A pH greater than 7 is basic (litmus is blue) The pH scale ranges from below zero (very acidic) to above 14 (very basic)
- 4. The pH scale is not linear. The pH scale is logarithmic. pH = -log[H+] [H+] = 1.0 x 10-2 pH = 2 very acidic [H+] = 1.0 x 10-3 pH = 3 acidic A solution with pH of 2 contains 10 times as much H+ as a solution with pH of 3.
- 5. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M [H+] = 5.0 x 10-5M
- 6. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M
- 7. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M pH is between 4&5
- 8. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M pH is between 4&5 to CHECK with calculator 1.Enter concentration in calculator 2.Press log key 3.Change sign to positive OR in the reverse order depending on your calculator!
- 9. Calculate [H+] given pH pH = 7.0 pH= 8.5
- 10. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5
- 11. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5 [H+]= 3.2x10-9
- 12. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5 [H+]= 3.2x10-9 = between 1x10-9 and 1x10-8 1. Enter pH value in calculator 2. Press the +/- key 3. Press the 10x key OR in the reverse order depending on your calculator!
- 13. From pH0 to pH14 the H+ concentration decreases 100,000,000,000,000 times!!
- 14. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 ALWAYS!!
- 15. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 ALWAYS!! If [H+] = 1.0 x 10-5 then, [OH-] = 1.0x10-14/ 1.0x10-5 = 1 x 10-9 SUBTRACT THE EXPONENTS!
- 16. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 If [H+] = 5.0 x 10-4 then, [OH-] = 1.0x10-14/ 5.0x10-4 = 2 x 10-11
- 17. Acidic = more H+ than OH- Basic = more OH- than H+ There is always some of each ion in water no matter what the pH
- 19. Relationship between pH and pOH pOH = -log[OH-] pH + pOH = 14 Always!!
- 20. pH pH + pOH = 14 pOH pH = pOH = -log[H+] -log[OH-] [H+] [H+][OH-]=1x10-14 [OH-]
- 21. pH pH + pOH = 14 pOH [H+] = [OH-]= 10-pH 10-pOH [H+] [H+][OH-]=1x10-14 [OH-]
- 22. Calculate pH ↔ pOH What is the pH of a solution with pOH = 13? Is the solution acidic or basic? What is the pOH of a solution with pH = 8? Is the solution acidic or basic? What are the concentrations of H+ and OH-?
- 23. Calculate pH ↔ pOH What is the pH of a solution with pOH = 13? Is the solution acidic or basic? pH= 1, acidic What is the pOH of a solution with pH = 8? Is the solution acidic or basic? pOH = 6, basic What are the concentrations of H+ and OH-? [H+] = 1.0x10-1, [OH-] = 1.0x10-13 [H+] = 1.0x10-8, [OH-] = 1.0x10-6