Endothermic and exothermic process (Data Logging)
•Descargar como PPT, PDF•
0 recomendaciones•2,334 vistas
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
Steam condensers - Part 5 (Air leakage and Condenser Performance)

Steam condensers - Part 5 (Air leakage and Condenser Performance)
Destacado
Destacado (19)
Endothermic and Exothermic Reactions Lesson PowerPoint

Endothermic and Exothermic Reactions Lesson PowerPoint
Similar a Endothermic and exothermic process (Data Logging)
Similar a Endothermic and exothermic process (Data Logging) (20)
Notes for Unit 17 of AP Chemistry (Thermodynamics)

Notes for Unit 17 of AP Chemistry (Thermodynamics)
Endothermic and exothermic process (Data Logging)
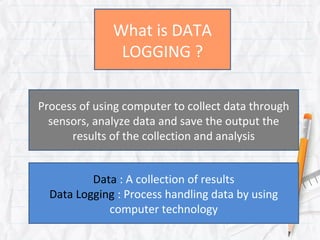
- 1. What is DATA LOGGING ? Process of using computer to collect data through sensors, analyze data and save the output the results of the collection and analysis Data : A collection of results Data Logging : Process handling data by using computer technology
- 2. ELEMENTS OF DATA LOGGING
- 4. Endothermic and exothermic process
- 5. engage a b
- 7. Empower Sensor Data collector
- 9. Result Solution of copper (II) sulphate,
- 11. Solution of anhydrous copper (II) sulphate
- 13. • Is the enthalpy change for this reaction exothermic or endothermic? • What sign should the enthalpy change have? • What is the equation for this reaction? • What bonds are being broken and formed in this reaction? • What is this enthalpy change called?
- 14. • How can we make a triangle between these reactions?
- 15. Enhance Cold packs and putting ice in towel causes a cooling effect on their person’s head and temporarily relieve the pain and fever. Explain.
- 16. a) Cold Pack • When the cold pack is used, the chemicals inside the pack are made to react with each other and this reaction is highly endothermic in nature. • Endothermic reactions involve the absorption of heat.
- 17. • The ammonium nitrate mixing with the water creates cold. The temperature of cold packs can reach back to normal temperature. • The heat energy is taken into the system from the surrounding. The surrounding in this case is the person’s head.
- 18. Extension Production of Ammonia Gas : N2 (g) + 3H2 (g) ↔ 2NH3 (g) What will happen to the reaction if we increase the concentration the temperature of the reactant mixture?
- 19. • If the temperature of a reaction mixture is increase, the equilibrium will shift to decrease the temperature. • Based on Le Chatelier’s Principle which stated that if a chemical system at equilibrium experiences a change in concentration, temperature or total pressure, the equilibrium will shift in order to minimize that changes a new equilibrium is established.
- 20. • So, if we increase the temperature, the equilibrium will shift to the reactant part which is left. • So, the reaction will undergo endothermic reaction as it use up heat energy. • Ammonia will broken down into hydrogen and nitrogen gas. An increase in temperature will decrease the yield of ammonia , NH3.