Revision on consumer, r te, thermo and carbon compound
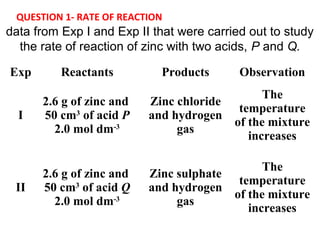
- 1. Exp Reactants Products Observation I 2.6 g of zinc and 50 cm3 of acid P 2.0 mol dm-3 Zinc chloride and hydrogen gas The temperature of the mixture increases II 2.6 g of zinc and 50 cm3 of acid Q 2.0 mol dm-3 Zinc sulphate and hydrogen gas The temperature of the mixture increases data from Exp I and Exp II that were carried out to study the rate of reaction of zinc with two acids, P and Q. QUESTION 1- RATE OF REACTION
- 2. • (i) By choosing either Experiment I or Experiment II, state the name of the acid used. •
- 3. • (i) Write the chemical equation for the reaction of this acid with zinc. [2 marks]
- 4. (ii) Draw an energy profile diagram for the reaction in 10(a)(i). On the energy profile diagram show the: –Heat of reaction, AH –Activation energy without a catalyst, –Activation energy with a catalyst, E' Explain the energy profile diagram. [10 marks]
- 5. ∆H E- activattion energy without catalyst E’- activattion energy with catalyst energy Energy profile diagram of reaction zinc and hydrochloric acid
- 6. (ii) Explain the energy profile diagram. ∆H
- 7. (ii) Explain the energy profile diagram. ∆H
- 8. (ii) Explain the energy profile diagram. ∆H ∆H
- 9. (ii) Explain the energy profile diagram. ∆H
- 10. (ii) Explain the energy profile diagram. ∆H
- 11. What is the function of these food additives? a) Colouring agents b) preservatives c) Anti oxidants d) Flavouring agents e) Stabilisers and thickeners a) To restore the colour of original food b) To destroy or prevent growth of micro organisms in food c) To prevention oxidation of food by air d) To to give or enhance the flavour of food e) To prevent food such as ice cream from separating into layers and to thicken soup or gravy Question no. 2) consumer
- 12. What type of food additives? Colouring agents preservatives Anti oxidants Flavouring agents Stabilisers and thickeners a) Benzoic acid b) Pectin c) Saccharin d) aspartam e) Ascorbic acid Flavouring agents f) tartrazine f) Sodium nitrite preservatives
- 13. What type of food additives? Colouring agents preservatives Anti oxidants Stabilisers and thickeners a) Azo compound b) Vitamin E c) Mono sodium glutamate d) agar-agar e) Gelatin Flavouring agents f) Sodium benzoate Stabilisers and thickeners g) Sulphur dioxide preservatives
- 14. What is the function of these traditional medicines? a) lime b) garlic c) ginger d) onions e) Betel leaves- daun sireh a) To increase digestive juice b) Has antibiotic property c) To warm the body d) To purift blood e) Stop bleeding nose
- 15. What is the function of these modern medicines? a) analgesics b) antibiotics c) Psychotherapeutic medicines a) To relieve pain without affecting consciousness b) Kill bacteria c) To treat mental illness
- 16. a) stimulants b) Anti depressant c) Antipsychotic drug a) To stimulate mental activity b) To control worry, fear, anxiety, panic c) To treat psychotic illness or schizophrenia c) Psychotherapeutic medicines
- 17. What type of modern medicines? analgesics Anti biotics a) aspirin b) Penicillin c) streptomycin d) Para cetamol e) caffeine f) codeine g) barbiturate Anti biotics analgesics stimulant analgesics antidepressant h) tranquiliser antidepressant
- 18. What is the uses of modern medicines? Fever, prevent heart attack fever a) aspirin b) Penicillin c) streptomycin d) Para cetamol f) codeine pneumonia Tuberculosis, dysentry Treat gonorrhea, syphlis, pneumonia, meningitis Cough medicine, headache
- 19. Soap: RCOO – Na + or R COO – K + Detergent: What is general formula for soap? and detergent?
- 20. What does hard water contains? •Hard water contains calcium ion, Ca2+ or magnesium ion, Mg2+.
- 21. Why do soaps form scum with hard water? • Soap reacts with calcium ion, Ca2+ or magnesium ion, Mg2+ in hard water to form insoluble salt, called scum
- 22. Why is soap not effective in hard water? • Because formation of scum reduce the amount of soap for cleaning and thus wastage of soap occur.
- 23. Why is detergent more effective than soap in hard water? • Detergent form soluble salt with calcium ion, Ca2+ or magnesium ion, Mg2+ in hard water. • Therefore the cleaning power is not affected by hard water.
- 24. Below are some additives in detergents. Fill in missing words Additives Functions 1 Drying agents ( such as sodium sulphate, sodium silicate) to keep the powder……… ………. DRY
- 25. Additives Functions 2 Builders ( such as Sodium triphosphate) …………… water. softe n
- 26. Additives Functions 3 Biological enzymes ( such as amylase, lipase) to digest in dirt or to modify fabric feel PROTEIN, FAT OR CARBOHYDRA TE
- 27. Additives Functions 4 Such as sodium perborate Convert stains into ………………… substances colourles s
- 28. Additives Functions 5 Perfumes Make clothes smell ……………. And clean fres h
- 29. Additives Functions 6. Stabilizers ( such as silicones) Prevent formation of ………………foam
- 30. Change of one homologous series to another homologous series Question 3) Revision on alcohols, carboxylic acids and ester
- 37. Alcohol Carboxylic acid Ester State the general formula for
- 38. Many reactions of ethanol
- 39. Reaction 1 • Combustion • ethanol is burnt in excess oxygen Observation: ethanol burns with blue flame, no soot
- 40. Reaction 2 • Dehydration • ethanol is passed over heated porcelain chips to produce ethene Observation: gas formed that will decolorize brown bromine water
- 41. Reaction 3 • hydration or addition of steam • When a mixture of ethene and steam is heated over phosphoric acid as catalyst, temp of 300o C, pressure 60 atm.
- 42. Reaction 4 • Esterification • When a mixture of ethanol and ethanoic acid is warmed with conc sulphuric acid as catalyst, Observation: sweet smelling liquid is formed
- 43. Reaction 5 • Esterification • When a mixture of ethanoic acid and ethanol is warmed with conc sulphuric acid as catalyst Observation: Sweet smelling liquid is formed
- 44. Reaction 6 • Oxidation • When ethanol is warmed with acidified potassium dichromate as oxidizing agent Observation: Orange solution change to green
- 45. Naming of esters
- 46. 5) A student obtained the data to determine heat of precipitation of PbSO4 Solution Vol (cm3) Conc (moldm- 3) Initial temp (0 C) Pb(NO3)2 50 0.5 27.4 Na2SO4 50 0.5 27.6 Highest temperature : 30.5 0 C Question 4- thermochemistry
- 47. What is meant by heat of precipitation? • Heat changed when 1 mole of precipitate is formed from its ions in an aqueous solution
- 48. b) Calculate heat of precipitation of PbSO4 • Mole Pb 2+ : (0.5)(50)/1000 = 0.025 mol • Mole SO4 2- : (0.5)(50)/1000 = 0.025 mol • Pb2+ + SO2- 4 PbSO4 mole PbSO4 = Mole Pb2+ or mole SO2- 4 = 0.025 mol
- 49. ϴ = 30.5 - ( 27.4 + 27.6 ) 2 = 3.0 ° C Heat of precipitation = mCϴ mol = ( 100)(4.2)(3) 0.025 = 50400 J/mol ∆ H = - 50.4 KJ/mol
- 50. d)Write thermo chemical equation •Pb2+ + SO4 2- PbSO4 ∆ H = - 50.4 KJ/mol
- 51. e) Write the ionic equation •Pb2+ + SO4 2- PbSO4
- 52. E) Construct energy level diagram Pb2+ + SO4 2- Energy ∆ H = - 50.4 KJ/mol PbSO4
- 53. f) The experiment is repeated using K2SO4 to replaced Na2SO4. Heat of precipitation of PbSO4 remain the same. Explain. • Because the same precipitate is formed, which is PbSO4. • Only Pb 2+ ions and SO4 2- ions react • Na+ ions and K+ ions do not react
- 54. • 6) A student carried out an experiment to determine heat of displacement of copper from CuSO4 solution. He added excess zinc powder to 50 cm3 of 0.2 moldm-3 CuSO4. The thermo chemical equation is shown below : Zn + Cu2+ Zn2+ + Cu ∆ H = -80.64 KJ/mol
- 55. a) Calculate the change in temperature Mol Copper= mol copper(II) sulphate = (0.2)(50)/1000 = 0.01 mol ∆ H = mCϴ mol 80 640 J = ( 50)(4.2)(ϴ) 0.01 ϴ = 3.8 ° C
- 56. b) Write the ionic equation Zn + CuSO4 Cu + ZnSO4 Zn + Cu2+ Zn2+ + Cu
- 57. The experiment is repeated with the following changes. What is the effect in the change of temperature when : • Concentration of CuSO4 is doubled, without changing the volume : So, change of temp or ϴ is doubled. Because as the concentration doubled, the number of particle per unit volume also doubled.
- 58. The experiment is repeated with the following changes. What is the effect in the change of temperature when : • volume of CuSO4 is halved, without changing the concentration : So, change of temp or ϴ is remain the same. Because, the changes in volume do not affect the number of particles per unit volume
- 59. 7) State the diff betw heat change and the heat of reaction HEAT CHANGE HEAT OF REACTION A) OTHER NAMES Depends on name of reactions : Heat absorb , heat released -Heat of Precipitation -Heat of displacement -Heat of neutralisation -Heat of combustion
- 60. 7) State the differences between heat change and heat of reaction HEAT CHANGE HEAT OF REACTION B) FORMULA USED H= mCϴ ∆ H= mCϴ mol C)UNIT Joule Kilo Joule/ mol D) SYMBOL none ∆ H E) SIGN No sign Either + for endothermic rex or – for exothermic rex
