Chemistry of alcohol
•Descargar como PPTX, PDF•
4 recomendaciones•3,008 vistas
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (19)
Chloroform - Formula, Preparation, Properties, Uses, History & Facts

Chloroform - Formula, Preparation, Properties, Uses, History & Facts
Thiazole - Synthesis of Thiazole - Reactions of Thiazole - Medicinal uses of ...

Thiazole - Synthesis of Thiazole - Reactions of Thiazole - Medicinal uses of ...
Destacado
Destacado (20)
Organic Chemistry: Carbonyl Compounds and Nitrogen Compounds

Organic Chemistry: Carbonyl Compounds and Nitrogen Compounds
21.1 - Part 1 Structure and Properties of Carboxylic Acid Derivatives - Wade 7th

21.1 - Part 1 Structure and Properties of Carboxylic Acid Derivatives - Wade 7th
Similar a Chemistry of alcohol
Similar a Chemistry of alcohol (20)
a. Isoamyl alcohol is an organic compound having .pdf

a. Isoamyl alcohol is an organic compound having .pdf
Chapter4alcoholsphenolsandethers 151111010603-lva1-app6892

Chapter4alcoholsphenolsandethers 151111010603-lva1-app6892
Unveiling the Secrets of Ethanol: Ethyl Alcohol and Organic Ethanol

Unveiling the Secrets of Ethanol: Ethyl Alcohol and Organic Ethanol
Chemistry of alcohol
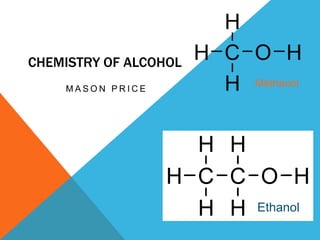
- 1. CHEMISTRY OF ALCOHOL M A S O N P R I C E Methanol Ethanol
- 2. WHAT DO WE MEAN WHEN WE SAY ALCOHOL? • Alcohol – any organic compound containing OH- • Alcohol is used in fuel, paint, disinfectants, plastics and even beverages. • Alcohol usually refers to either ethanol or methanol, the most commonly used members of the alcohol family.
- 3. METHANOL CH3OH • Methanol is extremely toxic to humans and also very flammable. • If ingested, it causes hypoxia (lack of oxygen) in the cells. • It is used as a denaturant for other alcohols. • Also used as an additive for gasoline.
- 4. DENATURED ALCOHOL • Alcohol not intended for human consumption is denatured. • Denaturation is the process through which alcohol is rendered unfit for human consumption for tax reasons. • A toxic or extremely undesirable tasting liquid is added to make it impossible for people to drink. • Ethanol is denatured before it is used for chemical purposes.
- 5. ETHANOL C2-H5OH • Ethanol is the most common alcohol, found in beverages, paints, adhesives, and nail polish remover. • Diesel cars can run on 100% ethanol and all cars can run on a combination of gasoline and ethanol.
- 6. ISOPROPYL ALCOHOL C3H8O • Structural isomer of propyl alcohol. • Simplest of the secondary alcohols, which have the OH- attached to the second Carbon. • Known mostly as “rubbing alcohol”.
- 7. FERMENTATION/DISTILLATION • Ethyl alcohol is produced through fermentation of a grain, typically corn. • Methanol was produced through distillation of wood. • Methanol is now produced using water and a nickel catalyst according to the equation CH4 + H20 CO+ 3H2.
- 8. WORKS CITED "Alcohol." Chemistry Daily. Wikipedia, n.d. Web. 11 Apr. 2013. <http://www.chemistrydaily.com/chemistry/Alcohol>. Breedlove, C.H. "Denatured Alcohol." Chemmatters 14 Dec. 1990: 14-15. Print. "Ethyl Alcohol." Chemistry Daily. Wikipedia, n.d. Web. 11 Apr. 2013. <http://www.chemistrydaily.com/chemistry/Ethanol>. Goldsmith, Robert H. "Alcohol." Chemmatters 8 Feb. 1985: 8-11. Print. Methanol. The Chemical Company. The Chemical Company, n.d. Web. 28 Apr. 2013. <http://www.thechemco.com/chemical/methanol/>.