Organic Chemistry Chapter 21 Klein
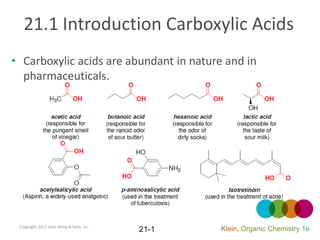
- 1. 21.1 Introduction Carboxylic Acids • Carboxylic acids are abundant in nature and in pharmaceuticals. Copyright 2012 John Wiley & Sons, Inc. 21-1 Klein, Organic Chemistry 1e
- 2. 21.1 Introduction Carboxylic Acids • The US produces over 2.5 million tons of acetic acid per year, which is primarily used to produce vinyl acetate. – Vinyl acetate is used in paints and adhesives. • Carboxylic acid derivatives, such as vinyl acetate, are very common, and they play a central role in organic chemistry. Copyright 2012 John Wiley & Sons, Inc. 21-2 Klein, Organic Chemistry 1e
- 3. 21.2 Nomenclature of Carboxylic Acids • Monocarboxylic acids are named with the suffix “oic acid.” • The carbon of the carboxylic acid moiety is assigned the locant position 1. Copyright 2012 John Wiley & Sons, Inc. 21-3 Klein, Organic Chemistry 1e
- 4. 21.2 Nomenclature of Carboxylic Acids • When the carboxylic acid group is attached to a ring, it is named as an alkane carboxylic acid. • There are also many common names for carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. 21-4 Klein, Organic Chemistry 1e
- 5. 21.2 Nomenclature of Carboxylic Acids • Dicarboxylic acids are named with the suffix “dioic acid.” • There are also many common names for dicarboxylic acids: • Practice with CONCEPTUAL CHECKPOINTs 12.1 through 12.3. Copyright 2012 John Wiley & Sons, Inc. 21-5 Klein, Organic Chemistry 1e
- 6. 21.3 Structure and Properties of Carboxylic Acids • The carbon atom of the carboxylic acid has a trigonal planar geometry. WHY? • The acid moiety is capable of strong hydrogen (H-) bonding including H-bonding between acid pairs. • As a result, carboxylic acids generally have high boiling points. – Consider the BPs of acetic acid (118 °C) and isopropanol (82 °C). Copyright 2012 John Wiley & Sons, Inc. 21-6 Klein, Organic Chemistry 1e
- 7. 21.3 Structure and Properties of Carboxylic Acids • Carboxylate ions end in the suffix “oate.” • Compounds that end in the suffix “oate” are often found in food ingredient lists as preservatives. • NaOH is a strong base, so it is capable of reacting ≈100% with a carboxylic acid. Copyright 2012 John Wiley & Sons, Inc. 21-7 Klein, Organic Chemistry 1e
- 8. 21.3 Structure and Properties of Carboxylic Acids • In water, the equilibrium generally favors the acid . • pKa values mostly range between 4 and 5. What is pKa? Copyright 2012 John Wiley & Sons, Inc. 21-8 Klein, Organic Chemistry 1e
- 9. 21.3 Structure and Properties of Carboxylic Acids • How does the pKa value for a carboxylic acid compare to a strong acid like HCl, or a very weak acid like ethanol? H–Cl pKa = -7 • How can induction and resonance be used to explain the acidity of a carboxylic acid? • Practice with CONCEPTUAL CHECKPOINTs 21.4 through 21.7. Copyright 2012 John Wiley & Sons, Inc. 21-9 Klein, Organic Chemistry 1e
- 10. 21.3 Structure and Properties of Carboxylic Acids • Let’s examine the equilibrium between the carboxylic acid and the carboxylate at physiological pH (7.3). • The acid and the conjugate base make a buffer. HOW? • Recall that the Henderson-Hasselbalch equation can be used to calculate the pH of a buffer: • Assuming the pKa is 4.3, calculate the ratio of carboxylate/acid. Copyright 2012 John Wiley & Sons, Inc. 21-10 Klein, Organic Chemistry 1e
- 11. 21.3 Structure and Properties of Carboxylic Acids • Many biomolecules exhibit carboxylic acid moieties. • Biomolecules such as pyruvic acid exist primarily as the carboxylate under physiological conditions. • Practice with CONCEPTUAL CHECKPOINT 21.8. Copyright 2012 John Wiley & Sons, Inc. 21-11 Klein, Organic Chemistry 1e
- 12. 21.3 Structure and Properties of Carboxylic Acids • Electron withdrawing substituents have a great effect on acidity. • WHY? Copyright 2012 John Wiley & Sons, Inc. 21-12 Klein, Organic Chemistry 1e
- 13. 21.3 Structure and Properties of Carboxylic Acids • Electron withdrawing substituents affect benzoic acid as well. • Practice with CONCEPTUAL CHECKPOINT 21.9. Copyright 2012 John Wiley & Sons, Inc. 21-13 Klein, Organic Chemistry 1e
- 14. 21.4 Preparation of Carboxylic Acids • In earlier chapters, we already learned some methods to synthesize carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. 21-14 Klein, Organic Chemistry 1e
- 15. 21.4 Preparation of Carboxylic Acids • In earlier chapters, we already learned some methods to synthesize carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. 21-15 Klein, Organic Chemistry 1e
- 16. 21.4 Preparation of Carboxylic Acids • Let’s examine two more ways to make carboxylic acids: 1. The hydrolysis of a nitrile can produce a carboxylic acid. – The mechanism will be discussed later. – Carboxylic acids can be made from alkyl halides using a two- step process. Copyright 2012 John Wiley & Sons, Inc. 21-16 Klein, Organic Chemistry 1e
- 17. 21.4 Preparation of Carboxylic Acids • Let’s examine two more ways to make carboxylic acids: 2. Carboxylation of a Grignard reaction can be achieved using CO2. – The Grignard reagent and the H3O+ cannot be added together. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-17 Klein, Organic Chemistry 1e
- 18. 21.4 Preparation of Carboxylic Acids • This gives us a second method to convert an alkyl halide into a carboxylic acid: • Practice with CONCEPTUAL CHECKPOINT 12.10. Copyright 2012 John Wiley & Sons, Inc. 21-18 Klein, Organic Chemistry 1e
- 19. 21.5 Reactions of Carboxylic Acids • LiAlH4 (LAH) is a strong reducing agent that can convert an acid to a primary alcohol: – The LAH acts as a base first. – Then, an aldehyde is produced. Copyright 2012 John Wiley & Sons, Inc. 21-19 Klein, Organic Chemistry 1e
- 20. 21.5 Reactions of Carboxylic Acids • LiAlH4 (LAH) is a strong reducing agent that can convert an acid to a primary alcohol: – The aldehyde is further reduced to the alcohol. – Can the reduction be stopped at the aldehyde? Copyright 2012 John Wiley & Sons, Inc. 21-20 Klein, Organic Chemistry 1e
- 21. 21.5 Reactions of Carboxylic Acids • The milder borane reagent can also be used to promote the reduction. • Reduction with borane is selective compared to LAH reduction. • Practice with CONCEPTUAL CHECKPOINT 21.11. Copyright 2012 John Wiley & Sons, Inc. 21-21 Klein, Organic Chemistry 1e
- 22. 21.6 Introduction to Carboxylic Acid Derivatives • The reduction of acids with LAH or borane result in a decrease in the oxidation number for carbon. HOW? • There are also many reactions where carboxylic acids don’t change their oxidation state. • What criteria must Z fulfill so that there is no change in the oxidation state? Copyright 2012 John Wiley & Sons, Inc. 21-22 Klein, Organic Chemistry 1e
- 23. 21.6 Introduction to Carboxylic Acid Derivatives • When Z is a heteroatom, the compound is called a carboxylic acid derivative. • Because it has the same oxidation state, a nitrile is also an acid derivative despite not having a carbonyl group. Copyright 2012 John Wiley & Sons, Inc. 21-23 Klein, Organic Chemistry 1e
- 24. 21.6 Introduction to Carboxylic Acid Derivatives • Acid halides and anhydrides are relatively unstable, so they are not common in nature; we will discuss their instability in detail later in this chapter. • Some naturally occurring esters are known to have pleasant odors: Copyright 2012 John Wiley & Sons, Inc. 21-24 Klein, Organic Chemistry 1e
- 25. 21.6 Introduction to Carboxylic Acid Derivatives • Amides are VERY common in nature. • What type of molecule in nature includes amide linkages? • Many other compounds feature amides, including some natural sedatives like melatonin. Copyright 2012 John Wiley & Sons, Inc. 21-25 Klein, Organic Chemistry 1e
- 26. 21.6 Introduction to Carboxylic Acid Derivatives • To name an acid halide, replace “ic acid” with “yl halide.” Copyright 2012 John Wiley & Sons, Inc. 21-26 Klein, Organic Chemistry 1e
- 27. 21.6 Introduction to Carboxylic Acid Derivatives • Alternatively, the suffix, “carboxylic acid” can be replaced with “carbonyl halide.” Copyright 2012 John Wiley & Sons, Inc. 21-27 Klein, Organic Chemistry 1e
- 28. 21.6 Introduction to Carboxylic Acid Derivatives • Acid anhydrides are named by replacing “acid” with “anhydride.” Copyright 2012 John Wiley & Sons, Inc. 21-28 Klein, Organic Chemistry 1e
- 29. 21.6 Introduction to Carboxylic Acid Derivatives • Asymmetric acid anhydrides are named by listing the acids alphabetically and adding the word anhydride. Copyright 2012 John Wiley & Sons, Inc. 21-29 Klein, Organic Chemistry 1e
- 30. 21.6 Introduction to Carboxylic Acid Derivatives • Esters are named by naming the alkyl group attached to the oxygen followed by the carboxylic acid’s name with the suffix “ate.” Copyright 2012 John Wiley & Sons, Inc. 21-30 Klein, Organic Chemistry 1e
- 31. 21.6 Introduction to Carboxylic Acid Derivatives • Amides are named by replacing the suffix “ic acid” or “oic acid” with “amide.” Copyright 2012 John Wiley & Sons, Inc. 21-31 Klein, Organic Chemistry 1e
- 32. 21.6 Introduction to Carboxylic Acid Derivatives • If the nitrogen atom of the amide group bears alkyl substituents, their names are placed at the beginning of the name with N as their locant. Copyright 2012 John Wiley & Sons, Inc. 21-32 Klein, Organic Chemistry 1e
- 33. 21.6 Introduction to Carboxylic Acid Derivatives • Nitriles are named by replacing the suffix “ic acid” or “oic acid” with “onitrile.” • Practice with CONCEPTUAL CHECKPOINTs 21.12 and 21.13. Copyright 2012 John Wiley & Sons, Inc. 21-33 Klein, Organic Chemistry 1e
- 34. 21.7 Reactivity of Carboxylic Acid Derivatives • In general, carboxylic acid derivatives are good electrophiles. • WHY? Copyright 2012 John Wiley & Sons, Inc. 21-34 Klein, Organic Chemistry 1e
- 35. 21.7 Reactivity of Carboxylic Acid Derivatives • Reactivity can be affected by – Induction – Resonance – Sterics – Quality of leaving group Copyright 2012 John Wiley & Sons, Inc. 21-35 Klein, Organic Chemistry 1e
- 36. 21.7 Reactivity of Carboxylic Acid Derivatives • Let’s examine the acid chloride: – The electronegative chlorine enhances the electrophilic character of the carbonyl. HOW? – There are 3 resonance contributors to the acid chloride: – The chlorine does not significantly donate electron density to the carbonyl. HOW does that affect its quality as an electrophile. Copyright 2012 John Wiley & Sons, Inc. 21-36 Klein, Organic Chemistry 1e
- 37. 21.7 Reactivity of Carboxylic Acid Derivatives • Let’s examine the acid chloride: – Describe how the presence of the chloride affects the sterics of the nucleophilic attack on the carbonyl. – The chloride is a good leaving group, which also enhances its reactivity. • Considering all of the factors involved, the acid chloride is quite reactive. Copyright 2012 John Wiley & Sons, Inc. 21-37 Klein, Organic Chemistry 1e
- 38. 21.7 Reactivity of Carboxylic Acid Derivatives • Amides are the least reactive acid derivative. • Examine the factors below to explain amide reactivity: – Induction – Resonance – Sterics – Quality of leaving group Copyright 2012 John Wiley & Sons, Inc. 21-38 Klein, Organic Chemistry 1e
- 39. 21.7 Reactivity of Carboxylic Acid Derivatives • Aldehydes and ketones are also electrophilic, but they do not undergo substitution. • WHY? Consider induction, resonance, sterics, and quality of leaving group. Copyright 2012 John Wiley & Sons, Inc. 21-39 Klein, Organic Chemistry 1e
- 40. 21.7 Reactivity of Carboxylic Acid Derivatives • Nucleophilic acyl substitution is a two-step process. – Because C=O double bonds are quite stable, the “loss of leaving group” step should occur if a leaving group is present. – – H and –R do not qualify as leaving groups. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-40 Klein, Organic Chemistry 1e
- 41. 21.7 Reactivity of Carboxylic Acid Derivatives • Let’s analyze a specific example: – The highest quality leaving group leaves the tetrahedral intermediate. Copyright 2012 John Wiley & Sons, Inc. 21-41 Klein, Organic Chemistry 1e
- 42. 21.7 Reactivity of Carboxylic Acid Derivatives • Do NOT draw the acyl substitution with an SN2 mechanism. • Sometimes a proton transfer will be necessary in the mechanism: – Under acidic conditions, (–) charges rarely form. WHY? – Under basic conditions, (+) charges rarely form. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-42 Klein, Organic Chemistry 1e
- 43. 21.7 Reactivity of Carboxylic Acid Derivatives • Under acidic conditions, (–) charges rarely form. – The first step will NOT be nucleophilic attack. – The electrophile and nucleophile are both low in energy. Copyright 2012 John Wiley & Sons, Inc. 21-43 Klein, Organic Chemistry 1e
- 44. 21.7 Reactivity of Carboxylic Acid Derivatives • H3O+ is unstable and drives the equilibrium forward by starting the reaction mechanism. • Now that the electrophile carries a (+) charge, it is much less stable (higher in energy). Complete the rest of the mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-44 Klein, Organic Chemistry 1e
- 45. 21.7 Reactivity of Carboxylic Acid Derivatives • Under basic conditions, (+) charges rarely form. • The OH– is the most unstable species in the reaction and drives the equilibrium forward. • Continue the rest of the mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-45 Klein, Organic Chemistry 1e
- 46. 21.7 Reactivity of Carboxylic Acid Derivatives • Neutral nucleophiles are generally less reactive, but they can still react if given enough time. • An intermediate with both (+) and (-) charges forms. • Intermediates with two (+) or two (–) charges are very unlikely to form. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-46 Klein, Organic Chemistry 1e
- 47. 21.7 Reactivity of Carboxylic Acid Derivatives • Depending on reaction conditions, UP TO THREE proton transfers may be necessary in the mechanism: • Draw a complete mechanism for the reaction below. – Will the reaction be reversible? – What conditions could be employed to favor products? • Practice with SKILLBUILDER 21.1. Copyright 2012 John Wiley & Sons, Inc. 21-47 Klein, Organic Chemistry 1e
- 48. 21.7 Reactivity of Carboxylic Acid Derivatives • Give necessary reaction conditions and a complete mechanism for the reaction below. • Describe how conditions could be modified to favor the products as much as possible. Copyright 2012 John Wiley & Sons, Inc. 21-48 Klein, Organic Chemistry 1e
- 49. 21.8 Preparation and Reaction of Acid Chlorides • Acid chlorides have great synthetic utility. WHY? • An acid chloride may form when an acid is treated with SOCl2. Copyright 2012 John Wiley & Sons, Inc. 21-49 Klein, Organic Chemistry 1e
- 50. 21.8 Preparation and Reaction of Acid Chlorides Copyright 2012 John Wiley & Sons, Inc. 21-50 Klein, Organic Chemistry 1e
- 51. 21.8 Preparation and Reaction of Acid Chlorides • The mechanism is more favored in the presence of a non-nucleophilic base like pyridine. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-51 Klein, Organic Chemistry 1e
- 52. 21.8 Preparation and Reaction of Acid Chlorides: HYDROLYSIS • To avoid an acid chloride being converted into an acid, it must be protected from moisture. Copyright 2012 John Wiley & Sons, Inc. 21-52 Klein, Organic Chemistry 1e
- 53. 21.8 Preparation and Reaction of Acid Chlorides: ALCOHOLYSIS • Often acid chlorides are used to synthesize esters. • Give a complete mechanism showing the role of pyridine in the mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-53 Klein, Organic Chemistry 1e
- 54. 21.8 Preparation and Reaction of Acid Chlorides: AMINOLYSIS • Often acid chlorides are used to synthesize amides. • Give a complete mechanism showing why TWO equivalents are used. Copyright 2012 John Wiley & Sons, Inc. 21-54 Klein, Organic Chemistry 1e
- 55. 21.8 Preparation and Reaction of Acid Chlorides • Acid chlorides can also be reduced using LAH: Copyright 2012 John Wiley & Sons, Inc. 21-55 Klein, Organic Chemistry 1e
- 56. 21.8 Preparation and Reaction of Acid Chlorides • Acid chlorides can also be reduced using LAH: – The acid must be added after the LAH has given adequate time to react completely. Copyright 2012 John Wiley & Sons, Inc. 21-56 Klein, Organic Chemistry 1e
- 57. 21.8 Preparation and Reaction of Acid Chlorides • To stop the aldehyde from being reduced to the alcohol, a bulky reducing agent can be used. • HOW does lithium tri(t-butoxy) aluminum hydride allow the reduction to be stopped at the aldehyde? Copyright 2012 John Wiley & Sons, Inc. 21-57 Klein, Organic Chemistry 1e
- 58. 21.8 Preparation and Reaction of Acid Chlorides • Acid chlorides can also be attacked by Grignard nucleophiles: Copyright 2012 John Wiley & Sons, Inc. 21-58 Klein, Organic Chemistry 1e
- 59. 21.8 Preparation and Reaction of Acid Chlorides • Two equivalents of the Grignard yield a 3° alcohol. Copyright 2012 John Wiley & Sons, Inc. 21-59 Klein, Organic Chemistry 1e
- 60. 21.8 Preparation and Reaction of Acid Chlorides • The Gilman reagent is another nucleophilic organometallic reagent that reacts readily with acid chlorides. • The C–Cu bond is less ionic than the C–Mg bond. WHY? • How does the ionic character of the bond affect the reactivity of the organometallic reagent? Copyright 2012 John Wiley & Sons, Inc. 21-60 Klein, Organic Chemistry 1e
- 61. 21.8 Preparation and Reaction of Acid Chlorides • Figure 21.9 illustrates the reactions of acid chlorides that we discussed. • Practice with CONCEPTUAL CHECKPOINTs 21.18 through 21.20. Copyright 2012 John Wiley & Sons, Inc. 21-61 Klein, Organic Chemistry 1e
- 62. 21.8 Preparation and Reaction of Acid Chlorides • Fill in necessary reagents for the reactions below. Copyright 2012 John Wiley & Sons, Inc. 21-62 Klein, Organic Chemistry 1e
- 63. 21.9 Preparation and Reactions of Acid Anhydrides • Acetic anhydride can be synthesized by heating 2 moles of acetic acid. • Why is so much heat needed to drive the equilibrium forward? • This process doesn’t work for most other acids because their structures cannot withstand such high temperatures. Copyright 2012 John Wiley & Sons, Inc. 21-63 Klein, Organic Chemistry 1e
- 64. 21.9 Preparation and Reactions of Acid Anhydrides • A more practical synthesis occurs when an acid chloride is treated with a carboxylate. • The –R groups attached to the anhydride do not have to be equivalent. Copyright 2012 John Wiley & Sons, Inc. 21-64 Klein, Organic Chemistry 1e
- 65. 21.9 Preparation and Reactions of Acid Anhydrides • Given that they both contain good quality leaving groups, how do you think the reactions of anhydrides compare to the reactions we already saw for chlorides? • Which has a better leaving group? WHY? Copyright 2012 John Wiley & Sons, Inc. 21-65 Klein, Organic Chemistry 1e
- 66. 21.9 Preparation and Reactions of Acid Anhydrides • Figure 21.10 shows how anhydrides can undergo many reactions analogous to those of acid chlorides. Copyright 2012 John Wiley & Sons, Inc. 21-66 Klein, Organic Chemistry 1e
- 67. 21.9 Preparation and Reactions of Acid Anhydrides • A non-nucleophilic weak base such as pyridine is not necessary when acid anhydrides react with a nucleophile. WHY? • When a nucleophile reacts with an anhydride, there will be a carboxylic acid byproduct. WHY? • Why is it often a disadvantage to have such a byproduct in a reaction? Copyright 2012 John Wiley & Sons, Inc. 21-67 Klein, Organic Chemistry 1e
- 68. 21.9 Preparation and Reactions of Acid Anhydrides • Acetic anhydride is often used to acetylate an amine or an alcohol. Copyright 2012 John Wiley & Sons, Inc. 21-68 Klein, Organic Chemistry 1e
- 69. 21.9 Preparation and Reactions of Acid Anhydrides • Practice with CONCEPTUAL CHECKPOINT 21.21. Copyright 2012 John Wiley & Sons, Inc. 21-69 Klein, Organic Chemistry 1e
- 70. 21.10 Preparation of Esters • Fischer esterification combines a carboxylic acid and an alcohol using an acid catalyst. Copyright 2012 John Wiley & Sons, Inc. 21-70 Klein, Organic Chemistry 1e
- 71. 21.10 Preparation of Esters • Each step of the Fischer esterification mechanism is equilibrium. • Under acidic conditions, (–) charges are avoided. Copyright 2012 John Wiley & Sons, Inc. 21-71 Klein, Organic Chemistry 1e
- 72. 21.10 Preparation of Esters • The overall Fischer esterification reaction is an equilibrium process. • How might you use Le Châtelier’s principle to favor products? – How might you use Le Châtelier's principle to favor reactants? • Is there an entropy difference that might be exploited? Copyright 2012 John Wiley & Sons, Inc. 21-72 Klein, Organic Chemistry 1e
- 73. 21.10 Preparation of Esters • Esters can also be prepared by treating an acid chloride with an alcohol—see Section 21.8. • What is the role of pyridine? • Why doesn’t pyridine act as a nucleophile? • Practice with CONCEPTUAL CHECKPOINTs 21.22 and 21.23. Copyright 2012 John Wiley & Sons, Inc. 21-73 Klein, Organic Chemistry 1e
- 74. 21.11 Reactions of Esters • Esters can undergo hydrolysis in the presence of aqueous hydroxide (SAPONIFICATION). • Predict the last steps in the mechanism. • To produce a carboxylic acid, H3O+ must be added at the end. WHY? Copyright 2012 John Wiley & Sons, Inc. 21-74 Klein, Organic Chemistry 1e
- 75. 21.11 Reactions of Esters • SAPONIFICATION is an equilibrium process. – Analyze the reversibility of each step in the mechanism. – How might you use Le Châtelier’s principle to favor products? – How might you use Le Châtelier’s principle to favor reactants? – Is there an entropy difference that might be exploited? • Soap is made through the saponification of triglycerides. EXPLAIN HOW. Copyright 2012 John Wiley & Sons, Inc. 21-75 Klein, Organic Chemistry 1e
- 76. 21.11 Reactions of Esters • Ester hydrolysis can be catalyzed under acidic conditions. • The carbonyl of the ester is protonated, and then a water acts as a nucleophile attacking the carbonyl carbon. • Draw out the complete mechanism. • Show how regeneration of H3O+ makes it catalytic. Copyright 2012 John Wiley & Sons, Inc. 21-76 Klein, Organic Chemistry 1e
- 77. 21.11 Reactions of Esters • Esters can also undergo aminolysis. • The overall equilibrium favors the amide formation. – Because of enthalpy or entropy? • The synthetic utility is limited because the process is slow and because there are more efficient ways to synthesize amides. Copyright 2012 John Wiley & Sons, Inc. 21-77 Klein, Organic Chemistry 1e
- 78. 21.11 Reactions of Esters • Esters can be reduced using reagents such as LAH: – Two equivalents of reducing agent are required. – Two alcohols are produced. • Draw a reasonable mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-78 Klein, Organic Chemistry 1e
- 79. 21.11 Reactions of Esters • LAH is a strong reducing agent, so a full reduction beyond the aldehyde to the alcohol cannot be avoided. • When performed at low temperature, reduction with DIBAH yields an aldehyde. HOW? Copyright 2012 John Wiley & Sons, Inc. 21-79 Klein, Organic Chemistry 1e
- 80. 21.11 Reactions of Esters • Esters can also react with Grignard reagents. • Two moles can be used to make a tertiary alcohol. Copyright 2012 John Wiley & Sons, Inc. 21-80 Klein, Organic Chemistry 1e
- 81. 21.11 Reactions of Esters • Esters can also react with Grignard reagents. • Two moles can be used to make a tertiary alcohol. • Practice with CONCEPTUAL CHECKPOINTs 21.24 and 21.25. Copyright 2012 John Wiley & Sons, Inc. 21-81 Klein, Organic Chemistry 1e
- 82. 21.11 Reactions of Esters • Give necessary reagents for the conversions below. Copyright 2012 John Wiley & Sons, Inc. 21-82 Klein, Organic Chemistry 1e
- 83. 21.12 Preparation and Reactions of Amides • Nylon is a polyamide. • Polyester is made similarly. HOW? Copyright 2012 John Wiley & Sons, Inc. 21-83 Klein, Organic Chemistry 1e
- 84. 21.12 Preparation and Reactions of Amides • Amides can be hydrolyzed with H3O+, but the process is slow and requires high temperature. • The mechanism is very similar to that for the hydrolysis of an ester. • Show a complete mechanism. • WHY is the process generally slow? Copyright 2012 John Wiley & Sons, Inc. 21-84 Klein, Organic Chemistry 1e
- 85. 21.12 Preparation and Reactions of Amides • Amides can be hydrolyzed with H3O+, but the process is slow and requires high temperature. • Should the equilibrium favor reactants or products? WHY? • Where does the NH4+ come from? • Amide hydrolysis can also be promoted with NaOH, although the process is very slow. Copyright 2012 John Wiley & Sons, Inc. 21-85 Klein, Organic Chemistry 1e
- 86. 21.12 Preparation and Reactions of Amides • LAH can reduce an amide to an amine. • The mechanism is quite different from the others we have seen in this chapter. • When the H- attacks, which is the best leaving group? Copyright 2012 John Wiley & Sons, Inc. 21-86 Klein, Organic Chemistry 1e
- 87. 21.12 Preparation and Reactions of Amides • The iminium is reduced with a second equivalent of hydride. • Practice with CONCEPTUAL CHECKPOINTs 21.26 through 21.28. Copyright 2012 John Wiley & Sons, Inc. 21-87 Klein, Organic Chemistry 1e
- 88. 21.13 Preparation and Reactions of Nitriles • When a 1° or 2° alkyl halide is treated with a cyanide ion, the CN– acts as a nucleophile in an SN2 reaction. • Nitriles can also be made by dehydrating an amide using a variety of reagents including SOCl2. Copyright 2012 John Wiley & Sons, Inc. 21-88 Klein, Organic Chemistry 1e
- 89. 21.13 Preparation and Reactions of Nitriles • What base might you use? Copyright 2012 John Wiley & Sons, Inc. 21-89 Klein, Organic Chemistry 1e
- 90. 21.13 Preparation and Reactions of Nitriles • An aqueous strong acid solution can be used to hydrolyze a nitrile. • In the mechanism, the nitrogen is protonated multiple times and water acts as a nucleophile. • Draw a complete mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-90 Klein, Organic Chemistry 1e
- 91. 21.13 Preparation and Reactions of Nitriles • Basic hydrolysis of a nitrile can also be achieved. • Which group in the reaction acts as a nucleophile? • Which group acts to protonate the nitrogen? • Draw a complete mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-91 Klein, Organic Chemistry 1e
- 92. 21.13 Preparation and Reactions of Nitriles • Nitriles can also react with Grignards. • After the nitrile is consumed, H3O+ is added to form an imine, which can be hydrolyzed with excess H3O+ (aq) to form a ketone. SHOW a mechanism. Copyright 2012 John Wiley & Sons, Inc. 21-92 Klein, Organic Chemistry 1e
- 93. 21.13 Preparation and Reactions of Nitriles • Similar to how carboxylic acids can be converted to alcohols using LAH (Section 21.5), nitriles can be converted to amines. • Practice with CONCEPTUAL CHECKPOINTs 21.29 through 21.31. Copyright 2012 John Wiley & Sons, Inc. 21-93 Klein, Organic Chemistry 1e
- 94. 21.14 Synthetic Strategies • When designing a synthesis, there are two general considerations that we make: 1. Is there a change in the CARBON SKELETON? 2. Is there a change in FUNCTIONAL GROUPS? • We have learned many new FUNCTIONAL GROUP TRANSFORMATIONs in this chapter. • Practice with SKILLBUILDER 21.2. Copyright 2012 John Wiley & Sons, Inc. 21-94 Klein, Organic Chemistry 1e
- 95. 21.14 Synthetic Strategies Copyright 2012 John Wiley & Sons, Inc. 21-95 Klein, Organic Chemistry 1e
- 96. 21.14 Synthetic Strategies • Give necessary reagents for the conversion below. Multiple steps will be necessary. Copyright 2012 John Wiley & Sons, Inc. 21-96 Klein, Organic Chemistry 1e
- 97. 21.14 Synthetic Strategies • There are 2 categories of bond-forming reactions: Copyright 2012 John Wiley & Sons, Inc. 21-97 Klein, Organic Chemistry 1e
- 98. 21.14 Synthetic Strategies • When forming new carbon-carbon bonds, it is critical to install functional groups in the proper location. • Give necessary reagents for the conversion below. More than one step will be necessary. • Practice with SKILLBUILDER 21.3. Copyright 2012 John Wiley & Sons, Inc. 21-98 Klein, Organic Chemistry 1e
- 99. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives • Recall that C=O stretching is a prominent peak in IR spectra. • Recall that conjugated carbonyl signals appear at lower wavenumbers (about 40 cm-1 less). Copyright 2012 John Wiley & Sons, Inc. 21-99 Klein, Organic Chemistry 1e
- 100. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives • The O–H stretch of an acid gives a very broad peak (2500-3300 cm-1). • The C N triple bond stretch appears around 2200 cm-1. • Carbonyl 13C peaks appear around 160-185 ppm. • Nitrile 13C peaks appear around 115-130 ppm. • The 1H peak for a carboxylic acid proton appears around 12 ppm. • Practice with CONCEPTUAL CHECKPOINT 21.38. Copyright 2012 John Wiley & Sons, Inc. 21-100 Klein, Organic Chemistry 1e
- 101. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives • Predict the number and chemical shift of all 13C peaks for the molecule below. • Predict the number, chemical shift, multiplicity, and integration of all 1H peaks for the molecule below. Copyright 2012 John Wiley & Sons, Inc. 21-101 Klein, Organic Chemistry 1e