Organic Chemistry II Ch 21 Klein
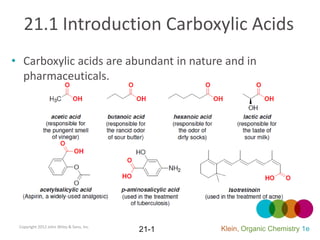
- 1. 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-1
- 2. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which is primarily used to produce vinyl acetate. Vinyl acetate is used in paints and adhesives. Carboxylic acid derivatives, such as vinyl acetate, are very common, and they play a central role in organic chemistry. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-2
- 3. 21.2 Nomenclature of Carboxylic Acids Monocarboxylic acids are named with the suffix “oic acid.” The carbon of the carboxylic acid moiety is assigned the locant position 1. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-3
- 4. 21.2 Nomenclature of Carboxylic Acids When the carboxylic acid group is attached to a ring, it is named as an alkane carboxylic acid. There are also many common names for carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-4
- 5. 21.2 Nomenclature of Carboxylic Acids Dicarboxylic acids are named with the suffix “dioic acid.” There are also many common names for dicarboxylic acids: Practice with CONCEPTUAL CHECKPOINTs 12.1 through 12.3. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-5
- 6. 21.3 Structure and Properties of Carboxylic Acids The carbon atom of the carboxylic acid has a trigonal planar geometry. WHY? The acid moiety is capable of strong hydrogen (H) bonding including H-bonding between acid pairs. As a result, carboxylic acids generally have high boiling points. Consider the BPs of acetic acid (118 °C) and isopropanol (82 °C). Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-6
- 7. 21.3 Structure and Properties of Carboxylic Acids Carboxylate ions end in the suffix “oate.” Compounds that end in the suffix “oate” are often found in food ingredient lists as preservatives. NaOH is a strong base, so it is capable of reacting ≈100% with a carboxylic acid. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-7
- 8. 21.3 Structure and Properties of Carboxylic Acids In water, the equilibrium generally favors the acid . pKa values mostly range between 4 and 5. What is pKa? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-8
- 9. 21.3 Structure and Properties of Carboxylic Acids How does the pKa value for a carboxylic acid compare to a strong acid like HCl, or a very weak acid like ethanol? H–Cl pKa= -7 How can induction and resonance be used to explain the acidity of a carboxylic acid? Practice with CONCEPTUAL CHECKPOINTs 21.4 through 21.7. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-9
- 10. 21.3 Structure and Properties of Carboxylic Acids Let’s examine the equilibrium between the carboxylic acid and the carboxylate at physiological pH (7.3). The acid and the conjugate base make a buffer. HOW? Recall that the Henderson-Hasselbalch equation can be used to calculate the pH of a buffer: Assuming the pKa is 4.3, calculate the ratio of carboxylate/acid. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-10
- 11. 21.3 Structure and Properties of Carboxylic Acids Many biomolecules exhibit carboxylic acid moieties. Biomolecules such as pyruvic acid exist primarily as the carboxylate under physiological conditions. Practice with CONCEPTUAL CHECKPOINT 21.8. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-11
- 12. 21.3 Structure and Properties of Carboxylic Acids Electron withdrawing substituents have a great effect on acidity. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-12
- 13. 21.3 Structure and Properties of Carboxylic Acids Electron withdrawing substituents affect benzoic acid as well. Practice with CONCEPTUAL CHECKPOINT 21.9. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-13
- 14. 21.4 Preparation of Carboxylic Acids In earlier chapters, we already learned some methods to synthesize carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-14
- 15. 21.4 Preparation of Carboxylic Acids In earlier chapters, we already learned some methods to synthesize carboxylic acids. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-15
- 16. 21.4 Preparation of Carboxylic Acids Let’s examine two more ways to make carboxylic acids: The hydrolysis of a nitrile can produce a carboxylic acid. The mechanism will be discussed later. Carboxylic acids can be made from alkyl halides using a two-step process. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-16
- 17. 21.4 Preparation of Carboxylic Acids Let’s examine two more ways to make carboxylic acids: Carboxylation of a Grignard reaction can be achieved using CO2. The Grignard reagent and the H3O+ cannot be added together. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-17
- 18. 21.4 Preparation of Carboxylic Acids This gives us a second method to convert an alkyl halide into a carboxylic acid: Practice with CONCEPTUAL CHECKPOINT 12.10. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-18
- 19. 21.5 Reactions of Carboxylic Acids LiAlH4 (LAH) is a strong reducing agent that can convert an acid to a primary alcohol: The LAH acts as a base first. Then, an aldehyde is produced. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-19
- 20. 21.5 Reactions of Carboxylic Acids LiAlH4 (LAH) is a strong reducing agent that can convert an acid to a primary alcohol: The aldehyde is further reduced to the alcohol. Can the reduction be stopped at the aldehyde? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-20
- 21. 21.5 Reactions of Carboxylic Acids The milder borane reagent can also be used to promote the reduction. Reduction with borane is selective compared to LAH reduction. Practice with CONCEPTUAL CHECKPOINT 21.11. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-21
- 22. 21.6 Introduction to Carboxylic Acid Derivatives The reduction of acids with LAH or borane result in a decrease in the oxidation number for carbon. HOW? There are also many reactions where carboxylic acids don’t change their oxidation state. What criteria must Z fulfill so that there is no change in the oxidation state? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-22
- 23. 21.6 Introduction to Carboxylic Acid Derivatives When Z is a heteroatom, the compound is called a carboxylic acid derivative. Because it has the same oxidation state, a nitrile is also an acid derivative despite not having a carbonyl group. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-23
- 24. 21.6 Introduction to Carboxylic Acid Derivatives Acid halides and anhydrides are relatively unstable, so they are not common in nature; we will discuss their instability in detail later in this chapter. Some naturally occurring esters are known to have pleasant odors: Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-24
- 25. 21.6 Introduction to Carboxylic Acid Derivatives Amides are VERY common in nature. What type of molecule in nature includes amide linkages? Many other compounds feature amides, including some natural sedatives like melatonin. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-25
- 26. 21.6 Introduction to Carboxylic Acid Derivatives To name an acid halide, replace “ic acid” with “yl halide.” Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-26
- 27. 21.6 Introduction to Carboxylic Acid Derivatives Alternatively, the suffix, “carboxylic acid” can be replaced with “carbonyl halide.” Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-27
- 28. 21.6 Introduction to Carboxylic Acid Derivatives Acid anhydrides are named by replacing “acid” with “anhydride.” Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-28
- 29. 21.6 Introduction to Carboxylic Acid Derivatives Asymmetric acid anhydrides are named by listing the acids alphabetically and adding the word anhydride. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-29
- 30. 21.6 Introduction to Carboxylic Acid Derivatives Esters are named by naming the alkyl group attached to the oxygen followed by the carboxylic acid’s name with the suffix “ate.” Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-30
- 31. 21.6 Introduction to Carboxylic Acid Derivatives Amides are named by replacing the suffix “ic acid” or “oic acid” with “amide.” Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-31
- 32. 21.6 Introduction to Carboxylic Acid Derivatives If the nitrogen atom of the amide group bears alkyl substituents, their names are placed at the beginning of the name with N as their locant. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-32
- 33. 21.6 Introduction to Carboxylic Acid Derivatives Nitriles are named by replacing the suffix “ic acid” or “oic acid” with “onitrile.” Practice with CONCEPTUAL CHECKPOINTs 21.12 and 21.13. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-33
- 34. 21.7 Reactivity of Carboxylic Acid Derivatives In general, carboxylic acid derivatives are good electrophiles. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-34
- 35. 21.7 Reactivity of Carboxylic Acid Derivatives Reactivity can be affected by Induction Resonance Sterics Quality of leaving group Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-35
- 36. 21.7 Reactivity of Carboxylic Acid Derivatives Let’s examine the acid chloride: The electronegative chlorine enhances the electrophilic character of the carbonyl. HOW? There are 3 resonance contributors to the acid chloride: The chlorine does not significantly donate electron density to the carbonyl. HOW does that affect its quality as an electrophile. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-36
- 37. 21.7 Reactivity of Carboxylic Acid Derivatives Let’s examine the acid chloride: Describe how the presence of the chloride affects the sterics of the nucleophilic attack on the carbonyl. The chloride is a good leaving group, which also enhances its reactivity. Considering all of the factors involved, the acid chloride is quite reactive. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-37
- 38. 21.7 Reactivity of Carboxylic Acid Derivatives Amides are the least reactive acid derivative. Examine the factors below to explain amide reactivity: Induction Resonance Sterics Quality of leaving group Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-38
- 39. 21.7 Reactivity of Carboxylic Acid Derivatives Aldehydes and ketones are also electrophilic, but they do not undergo substitution. WHY? Consider induction, resonance, sterics, and quality of leaving group. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-39
- 40. 21.7 Reactivity of Carboxylic Acid Derivatives Nucleophilic acyl substitution is a two-step process. Because C=O double bonds are quite stable, the “loss of leaving group” step should occur if a leaving group is present. – H and –R do not qualify as leaving groups. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-40
- 41. 21.7 Reactivity of Carboxylic Acid Derivatives Let’s analyze a specific example: The highest quality leaving group leaves the tetrahedral intermediate. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-41
- 42. 21.7 Reactivity of Carboxylic Acid Derivatives Do NOT draw the acyl substitution with an SN2 mechanism. Sometimes a proton transfer will be necessary in the mechanism: Under acidic conditions, (–) charges rarely form. WHY? Under basic conditions, (+) charges rarely form. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-42
- 43. 21.7 Reactivity of Carboxylic Acid Derivatives Under acidic conditions, (–) charges rarely form. The first step will NOT be nucleophilic attack. The electrophile and nucleophile are both low in energy. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-43
- 44. 21.7 Reactivity of Carboxylic Acid Derivatives H3O+ is unstable and drives the equilibrium forward by starting the reaction mechanism. Now that the electrophile carries a (+) charge, it is much less stable (higher in energy). Complete the rest of the mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-44
- 45. 21.7 Reactivity of Carboxylic Acid Derivatives Under basic conditions, (+) charges rarely form. The OH– is the most unstable species in the reaction and drives the equilibrium forward. Continue the rest of the mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-45
- 46. 21.7 Reactivity of Carboxylic Acid Derivatives Neutral nucleophiles are generally less reactive, but they can still react if given enough time. An intermediate with both (+) and (-) charges forms. Intermediates with two (+) or two (–) charges are very unlikely to form. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-46
- 47. 21.7 Reactivity of Carboxylic Acid Derivatives Depending on reaction conditions, UP TO THREE proton transfers may be necessary in the mechanism: Draw a complete mechanism for the reaction below. Will the reaction be reversible? What conditions could be employed to favor products? Practice with SKILLBUILDER 21.1. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-47
- 48. 21.7 Reactivity of Carboxylic Acid Derivatives Give necessary reaction conditions and a complete mechanism for the reaction below. Describe how conditions could be modified to favor the products as much as possible. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-48
- 49. 21.8 Preparation and Reaction of Acid Chlorides Acid chlorides have great synthetic utility. WHY? An acid chloride may form when an acid is treated with SOCl2. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-49
- 50. 21.8 Preparation and Reaction of Acid Chlorides Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-50
- 51. 21.8 Preparation and Reaction of Acid Chlorides The mechanism is more favored in the presence of a non-nucleophilic base like pyridine. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-51
- 52. 21.8 Preparation and Reaction of Acid Chlorides: HYDROLYSIS To avoid an acid chloride being converted into an acid, it must be protected from moisture. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-52
- 53. 21.8 Preparation and Reaction of Acid Chlorides: ALCOHOLYSIS Often acid chlorides are used to synthesize esters. Give a complete mechanism showing the role of pyridine in the mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-53
- 54. 21.8 Preparation and Reaction of Acid Chlorides: AMINOLYSIS Often acid chlorides are used to synthesize amides. Give a complete mechanism showing why TWO equivalents are used. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-54
- 55. 21.8 Preparation and Reaction of Acid Chlorides Acid chlorides can also be reduced using LAH: Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-55
- 56. 21.8 Preparation and Reaction of Acid Chlorides Acid chlorides can also be reduced using LAH: The acid must be added after the LAH has given adequate time to react completely. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-56
- 57. 21.8 Preparation and Reaction of Acid Chlorides To stop the aldehyde from being reduced to the alcohol, a bulky reducing agent can be used. HOW does lithium tri(t-butoxy) aluminum hydride allow the reduction to be stopped at the aldehyde? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-57
- 58. 21.8 Preparation and Reaction of Acid Chlorides Acid chlorides can also be attacked by Grignard nucleophiles: Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-58
- 59. 21.8 Preparation and Reaction of Acid Chlorides Two equivalents of the Grignard yield a 3° alcohol. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-59
- 60. 21.8 Preparation and Reaction of Acid Chlorides The Gilman reagent is another nucleophilic organometallic reagent that reacts readily with acid chlorides. The C–Cu bond is less ionic than the C–Mg bond. WHY? How does the ionic character of the bond affect the reactivity of the organometallic reagent? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-60
- 61. 21.8 Preparation and Reaction of Acid Chlorides Figure 21.9 illustrates the reactions of acid chlorides that we discussed. Practice with CONCEPTUAL CHECKPOINTs 21.18 through 21.20. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-61
- 62. 21.8 Preparation and Reaction of Acid Chlorides Fill in necessary reagents for the reactions below. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-62
- 63. 21.9 Preparation and Reactions of Acid Anhydrides Acetic anhydride can be synthesized by heating 2 moles of acetic acid. Why is so much heat needed to drive the equilibrium forward? This process doesn’t work for most other acids because their structures cannot withstand such high temperatures. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-63
- 64. 21.9 Preparation and Reactions of Acid Anhydrides A more practical synthesis occurs when an acid chloride is treated with a carboxylate. The –R groups attached to the anhydride do not have to be equivalent. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-64
- 65. 21.9 Preparation and Reactions of Acid Anhydrides Given that they both contain good quality leaving groups, how do you think the reactions of anhydrides compare to the reactions we already saw for chlorides? Which has a better leaving group? WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-65
- 66. 21.9 Preparation and Reactions of Acid Anhydrides Figure 21.10 shows how anhydrides can undergo many reactions analogous to those of acid chlorides. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-66
- 67. 21.9 Preparation and Reactions of Acid Anhydrides A non-nucleophilic weak base such as pyridine is not necessary when acid anhydrides react with a nucleophile. WHY? When a nucleophile reacts with an anhydride, there will be a carboxylic acid byproduct. WHY? Why is it often a disadvantage to have such a byproduct in a reaction? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-67
- 68. 21.9 Preparation and Reactions of Acid Anhydrides Acetic anhydride is often used to acetylate an amine or an alcohol. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-68
- 69. 21.9 Preparation and Reactions of Acid Anhydrides Practice with CONCEPTUAL CHECKPOINT 21.21. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-69
- 70. 21.10 Preparation of Esters Fischer esterification combines a carboxylic acid and an alcohol using an acid catalyst. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-70
- 71. 21.10 Preparation of Esters Each step of the Fischer esterification mechanism is equilibrium. Under acidic conditions, (–) charges are avoided. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-71
- 72. 21.10 Preparation of Esters The overall Fischer esterification reaction is an equilibrium process. How might you use Le Châtelier’s principle to favor products? How might you use Le Châtelier's principle to favor reactants? Is there an entropy difference that might be exploited? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-72
- 73. 21.10 Preparation of Esters Esters can also be prepared by treating an acid chloride with an alcohol—see Section 21.8. What is the role of pyridine? Why doesn’t pyridine act as a nucleophile? Practice with CONCEPTUAL CHECKPOINTs 21.22 and 21.23. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-73
- 74. 21.11 Reactions of Esters Esters can undergo hydrolysis in the presence of aqueous hydroxide (SAPONIFICATION). Predict the last steps in the mechanism. To produce a carboxylic acid, H3O+ must be added at the end. WHY? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-74
- 75. 21.11 Reactions of Esters SAPONIFICATION is an equilibrium process. Analyze the reversibility of each step in the mechanism. How might you use Le Châtelier’s principle to favor products? How might you use Le Châtelier’s principle to favor reactants? Is there an entropy difference that might be exploited? Soap is made through the saponification of triglycerides. EXPLAIN HOW. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-75
- 76. 21.11 Reactions of Esters Ester hydrolysis can be catalyzed under acidic conditions. The carbonyl of the ester is protonated, and then a water acts as a nucleophile attacking the carbonyl carbon. Draw out the complete mechanism. Show how regeneration of H3O+ makes it catalytic. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-76
- 77. 21.11 Reactions of Esters Esters can also undergo aminolysis. The overall equilibrium favors the amide formation. Because of enthalpy or entropy? The synthetic utility is limited because the process is slow and because there are more efficient ways to synthesize amides. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-77
- 78. 21.11 Reactions of Esters Esters can be reduced using reagents such as LAH: Two equivalents of reducing agent are required. Two alcohols are produced. Draw a reasonable mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-78
- 79. 21.11 Reactions of Esters LAHis a strong reducing agent, so a full reduction beyond the aldehyde to the alcohol cannot be avoided. When performed at low temperature, reduction with DIBAH yields an aldehyde. HOW? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-79
- 80. 21.11 Reactions of Esters Esters can also react with Grignard reagents. Two moles can be used to make a tertiary alcohol. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-80
- 81. 21.11 Reactions of Esters Esters can also react with Grignard reagents. Two moles can be used to make a tertiary alcohol. Practice with CONCEPTUAL CHECKPOINTs 21.24 and 21.25. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-81
- 82. 21.11 Reactions of Esters Give necessary reagents for the conversions below. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-82
- 83. 21.12 Preparation and Reactions of Amides Nylon is a polyamide. Polyester is made similarly. HOW? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-83
- 84. 21.12 Preparation and Reactions of Amides Amides can be hydrolyzed with H3O+, but the process is slow and requires high temperature. The mechanism is very similar to that for the hydrolysis of an ester. Show a complete mechanism. WHY is the process generally slow? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-84
- 85. 21.12 Preparation and Reactions of Amides Amides can be hydrolyzed with H3O+, but the process is slow and requires high temperature. Should the equilibrium favor reactants or products? WHY? Where does the NH4+ come from? Amide hydrolysis can also be promoted with NaOH, although the process is very slow. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-85
- 86. 21.12 Preparation and Reactions of Amides LAH can reduce an amide to an amine. The mechanism is quitedifferent from the others we have seen in this chapter. When the H- attacks, which is the best leaving group? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-86
- 87. 21.12 Preparation and Reactions of Amides The iminium is reduced with a second equivalent of hydride. Practice with CONCEPTUAL CHECKPOINTs 21.26 through 21.28. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-87
- 88. 21.13 Preparation and Reactions of Nitriles When a 1° or 2° alkyl halide is treated with a cyanide ion, the CN– acts as a nucleophile in an SN2 reaction. Nitriles can also be made by dehydrating an amide using a variety of reagents including SOCl2. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-88
- 89. 21.13 Preparation and Reactions of Nitriles What base might you use? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-89
- 90. 21.13 Preparation and Reactions of Nitriles An aqueous strong acid solution can be used to hydrolyze a nitrile. In the mechanism, the nitrogen is protonated multiple times and water acts as a nucleophile. Draw a complete mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-90
- 91. 21.13 Preparation and Reactions of Nitriles Basic hydrolysis of a nitrile can also be achieved. Which group in the reaction acts as a nucleophile? Which group acts to protonate the nitrogen? Draw a complete mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-91
- 92. 21.13 Preparation and Reactions of Nitriles Nitriles can also react with Grignards. After the nitrile is consumed, H3O+ is added to form an imine, which can be hydrolyzed with excess H3O+ (aq) to form a ketone. SHOW a mechanism. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-92
- 93. 21.13 Preparation and Reactions of Nitriles Similar to how carboxylic acids can be converted to alcohols using LAH (Section 21.5), nitriles can be converted to amines. Practice with CONCEPTUAL CHECKPOINTs 21.29 through 21.31. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-93
- 94. 21.14 Synthetic Strategies When designing a synthesis, there are two general considerations that we make: Is there a change in the CARBON SKELETON? Is there a change in FUNCTIONAL GROUPS? We have learned many new FUNCTIONAL GROUP TRANSFORMATIONsin this chapter. Practice with SKILLBUILDER 21.2. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-94
- 95. 21.14 Synthetic Strategies Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-95
- 96. 21.14 Synthetic Strategies Give necessary reagents for the conversion below. Multiple steps will be necessary. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-96
- 97. 21.14 Synthetic Strategies There are 2 categories of bond-forming reactions: Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-97
- 98. 21.14 Synthetic Strategies When forming new carbon-carbon bonds, it is critical to install functional groups in the proper location. Give necessary reagents for the conversion below. More than one step will be necessary. Practice with SKILLBUILDER 21.3. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-98
- 99. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives Recall that C=O stretching is a prominent peak in IR spectra. Recall that conjugated carbonyl signals appear at lower wavenumbers (about 40 cm-1 less). Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-99
- 100. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives The O–H stretch of an acid gives a very broad peak (2500-3300 cm-1). The CN triple bond stretch appears around 2200 cm-1. Carbonyl 13C peaks appear around 160-185 ppm. Nitrile 13C peaks appear around 115-130 ppm. The 1H peak for a carboxylic acid proton appears around 12 ppm. Practice with CONCEPTUAL CHECKPOINT 21.38. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-100
- 101. 21.15 Spectroscopy of Carboxylic Acids and Their Derivatives Predict the number and chemical shift of all 13C peaks for the molecule below. Predict the number, chemical shift, multiplicity, and integration of all 1H peaks for the molecule below. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1e 21-101
