IB Chemistry on Redox Design and Nernst Equation
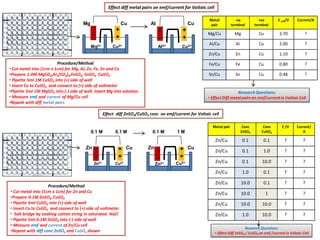
- 1. Research Questions: • Effect Diff ZnSO4 / CuSO4 on emf /current in Voltaic Cell Mg2+ Cu2+ Mg Cu Cu Cu2+ Zn Zn2+ Cu2+ CuAI AI3+ Zn Cu Zn2+ Cu2+ 0.1 M 0.1 M - - - - - - - - + + + + + + + + 0.1 M 1 M Effect diff metal pairs on emf/current for Voltaic cell Metal pair -ve terminal +ve terminal E cell/V Current/A Mg/Cu Mg Cu 2.70 ? AI/Cu AI Cu 2.00 ? Zn/Cu Zn Cu 1.10 ? Fe/Cu Fe Cu 0.80 ? Sn/Cu Sn Cu 0.48 ? Research Questions: • Effect Diff metalpairs on emf/current in Voltaic Cell Procedure/Method • Cut metal into (1cm x 1cm) for Mg, Al, Zn, Fe, Sn and Cu •Prepare 1.0M MgSO4,AI2(SO4)3,FeSO4, SnSO4, CuSO4 • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter •Pipette 5ml 1M MgSO4 into (-) side of well. Insert Mg into solution. • Measure emf and current of Mg/Cu cell •Repeat with diff metal pairs Metal pair Conc ZnSO4 Conc CuSO4 E /V Current/ A Zn/Cu 0.1 0.1 ? ? Zn/Cu 0.1 1.0 ? ? Zn/Cu 0.1 10.0 ? ? Zn/Cu 1.0 0.1 ? ? Zn/Cu 10.0 0.1 ? ? Zn/Cu 10.0 1 ? ? Zn/Cu 10.0 10.0 ? ? Zn/Cu 1.0 10.0 ? ? Effect diff ZnSO4/CuSO4 conc on emf/current for Voltaic cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu •Prepare 0.1M ZnSO4, CuSO4 • Pipette 5ml CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in saturated NaCI • Pipette 5ml 0.1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff conc ZnSO4 and CuSO4 shown
- 2. Zn2+ Cu2+ Zn Cu Cu Cu2+ Zn Zn2+ Cu2+ CuZn Zn2+ Zn Cu Zn2+ Cu2+ - - - - - - - - + + + + + + + + Effect diff ZnSO4 conc on emf/current for Voltaic cell Metal pair Conc ZnSO4 Conc CuSO4 E cell/V Current/ A Zn/Cu 10.0 1.0 ? ? Zn/Cu 1.0 1.0 ? ? Zn/Cu 0.1 1.0 ? ? Zn/Cu 0.01 1.0 ? ? Zn/Cu 0.001 1.0 ? ? Research Questions: • Effect Diff ZnSO4 conc on emf /current in Voltaic Cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in saturated NaCI • Pipette 5ml 10M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff conc ZnSO4 shown 10 M 1 M 1 M 1 M Effect diff CuSO4 conc 0n emf /current for Voltaic cell Metal pair Conc ZnSO4 Conc CuSO4 E cell/V Current/ A Zn/Cu 1.0 10.0 ? ? Zn/Cu 1.0 1.0 ? ? Zn/Cu 1.0 0.1 ? ? Zn/Cu 1.0 0.01 ? ? Zn/Cu 1.0 0.001 ? ? Research Questions: • Effect Diff CuSO4 conc on emf /current in Voltaic Cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 10M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in saturated NaCI • Pipette 5ml 1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff conc CuSO4 shown 1 M 10 M 1 M 1 M
- 3. Effect diff surface area/electrode size on emf/current for Voltaic cell Zn2+ Cu2+ Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in 1% NaCI • Pipette 5ml 1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff NaCI conc (salt bridge) shown. Effect diff salt bridge conc on emf /current for Voltaic cell Zn Cu Cu Cu2+ Zn Zn2+ Conc NaCI (salt bridge) Conc ZnSO4 Conc CuSO4 E /V Current /A Zn/Cu (1.0%) 1.0 1.0 ? ? Zn/Cu (2.0%) 1.0 1.0 ? ? Zn/Cu (3.0%) 1.0 1.0 ? ? Zn/Cu (4.0%) 1.0 1.0 ? ? Zn/Cu (5.0%) 1.0 1.0 ? ? Research Questions: • Effect Diff salt bridge conc on emf /current in Voltaic Cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in saturated NaCI • Pipette 5ml 1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with electrode size/surface area shown Metal pair Zn size Cu size E cell/V Current/A Zn/Cu 1 x 1 1 x 1 ? ? Zn/Cu 2 x 2 2 x 2 ? ? Zn/Cu 4 x 4 4 x 4 ? ? Zn/Cu 8 x 8 8 x 8 ? ? Zn/Cu 16 x 16 16 x 16 ? ? Research Questions: • Effect Diff surface area on emf /current in Voltaic Cell Cu2+ CuZn Zn2+ Zn Cu Zn2+ Cu2+ 1 % 2 % - - - - - - - - + + + + + + + +
- 4. Effect diff cation size/diffusion rate (salt bridge) on emf/current for Voltaic cell Zn2+ Cu2+ Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in 1% NaF • Pipette 5ml 1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff anion size (salt bridge) shown. Effect diff anion size/diffusion rate (salt bridge) on emf /current for Voltaic cell Zn Cu Cu Cu2+ Zn Zn2+ Research Questions: • Effect Diff anion size on emf /current in Voltaic Cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in 1% LiCI • Pipette 5ml 1M ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell •Repeat with diff cation size (salt bridge) shown Research Questions: • Effect Diff cation size on emf/current in Voltaic Cell Cu2+ CuZn Zn2+ Zn Cu Zn2+ Cu2+ Cation size (salt bridge) Conc ZnSO4 Conc CuSO4 E cell/V Current/ A Zn/Cu (LiCI) 1.0 1.0 ? ? Zn/Cu (NaCI) 1.0 1.0 ? ? Zn/Cu (KCI) 1.0 1.0 ? ? LiCI NaCI Anion size (salt bridge) Conc ZnSO4 Conc CuSO4 E cell/V Current/ A Zn/Cu (NaF) 1.0 1.0 ? ? Zn/Cu (NaCI) 1.0 1.0 ? ? Zn/Cu (NaBr) 1.0 1.0 ? ? Zn/Cu (NaI) 1.0 1.0 ? ? Zn/Cu (NaNO3) 1.0 1.0 ? ? Zn/Cu (NaSO4) 1.0 1.0 ? ? NaCINaF - - - - - - + + + + + + - - + +
- 5. Effect Temp on emf/current for Voltaic cell Zn2+ Cu2+ Procedure/Method • Cut metal into (1 x 1) for Cu and Cu •Prepare 1M CuSO4, CuSO4 • Pipette 5ml 1M CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter • Salt bridge by soaking cotton string in 1% NaCI • Pipette 5ml 1M CuSO4 into (-) side of well • Measure emf and current of Cu/Cu cell •Repeat with diff CuSO4 conc shown. Effect diff CuSO4 conc on emf/current for Copper Conc cell Zn Cu Cu Cu2+ Cu Cu2+ Research Questions: • Effect Diff CuSO4 conc on emf /current in Conc Cell Procedure/Method • Cut metal into (1cm x 1cm) for Zn and Cu •Prepare 1.0M ZnSO4, CuSO4 at 25C • Pipette 5ml CuSO4 into (+) side of well • Insert Cu to CuSO4 and connect to (+) side of voltmeter •Pipette 5ml ZnSO4 into (-) side of well • Measure emf and current of Zn/Cu cell at 25C •Repeat with diff temp shown Research Questions: • Effect Temp on emf/current in Voltaic Cell Cu2+ CuZn Zn2+ Cu Cu Cu2+ Cu2+ Temp/C Conc ZnSO4 Conc CuSO4 E cell/V Current/ A Zn/Cu (4oC) 1.0 1.0 ? ? Zn/Cu (25oC) 1.0 1.0 ? ? Zn/Cu (40oC) 1.0 1.0 ? ? Zn/Cu (50oC) 1.0 1.0 ? ? Zn/Cu (60oC) 1.0 1.0 ? ? 25oC 40oC Metal pair - ve Conc CuSO4 +ve Conc CuSO4 E /V Current/ A Cu/Cu 1.0 1.0 ? ? Cu/Cu 0.5 1.0 ? ? Cu/Cu 0.25 1.0 ? ? Cu/Cu 0.125 1.0 ? ? Cu/Cu 0.0625 1.0 ? ? 1 M 1 M - - - - - - - - + + + + + + + + 0.5 M 1 M
- 6. 1 ]1[ ]1[ ][ ][ 2 2 c c Q M M Cu Zn Q Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Find Eθ cell (use reduction potential) Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 1M 1M Zn2+ 1 M 1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) EE VE E E 10.1 010.1 )1ln( )965002( )29831.8( 10.1 Std condition 1M
- 7. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Find Eθ cell (use reduction potential) Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 1M 0.1M Zn2+ 10 ]1.0[ ]1[ ][ ][ 2 2 c c Q M M Cu Zn Q 0.1 M 1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) VE E E 07.1 03.010.1 )10ln( )965002( )29831.8( 10.1 Non std 0.1M E cell decrease ↓ [Cu2+] decrease ↓ ↓ Le Chatelier’s principle ↓ Cu2+ + 2e ↔ Cu ↓ [Cu2+] decrease ↓ ↓ Shift to left ← ↓ E cell → less ↓ +ve → Cu2+ less able ↓ to receive e- / Cu more able ↑ to lose e- [Cu2+] ↓ E cell < Eθ 1.07 < 1.10
- 8. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Find Eθ cell (use reduction potential) Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 1M 10M Zn2+ 1.0 ]10[ ]1[ ][ ][ 2 2 c c Q M M Cu Zn Q 10 M 1 M Using Nernst Eqn E0 =Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) VE E E 13.1 03.010.1 )1.0ln( )965002( )29831.8( 10.1 Non std 0.1M E cell increase ↑ [Cu2+] increase ↑ ↓ Le Chatelier’s principle ↓ Cu2+ + 2e ↔ Cu ↓ [Cu2+] increase ↑ ↓ Shift to right → ↓ E cell → more ↑ +ve → Cu2+ more able receive e- [Cu2+] ↑ E cell > Eθ 1.13 > 1.10
- 9. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Find Eθ cell (use reduction potential) Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 0.1M 1M Zn2+ 1.0 ]1[ ]1.0[ ][ ][ 2 2 c c Q M M Cu Zn Q 1 M 0.1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) VE E E 13.1 03.010.1 )1.0ln( )965002( )29831.8( 10.1 Non std 0.1M E cell increase ↑ [Zn2+] decrease ↓ ↓ Le Chatelier’s principle ↓ Zn2+ + 2e ↔ Zn ↓ [Zn2+] decrease ↓ ↓ Shift to left ← ↓ E cell → more ↑ +ve → Zn more able lose elec [Zn2+] ↓ E cell > Eθ 1.13 > 1.10
- 10. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Find Eθ cell (use reduction potential) Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 10M 1M Zn2+ 10 ]1[ ]10[ ][ ][ 2 2 c c Q M M Cu Zn Q 1 M 10 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) VE E E 07.1 03.010.1 )10ln( )965002( )29831.8( 10.1 Non std 10M E cell decrease ↓ [Zn2+] increase ↑ ↓ Le Chatelier’s principle ↓ Zn2+ + 2e ↔ Zn ↓ [Zn2+] increase ↑ ↓ Shift to right → ↓ E cell → less ↓ +ve → Zn less able ↓ lose e- [Zn2+] ↑ E cell < Eθ 1.07 < 1.10
- 11. Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Zn/Cu Cell -e -e Zn/Cu half cells Std electrode potential as std reduction potential Zn + Cu2+ → Zn2+ + Cu Eθ = ? Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn ↔ Zn2+ + 2e Eθ = +0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn - 0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu + 0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 1/2O2 + 2H+ +2e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.35 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F +2.87 +1.10 V Cu2+ - - - - Zn Cu + + + + Std condition 1M Q nF RT EE ln Zn +Cu2+→Zn2++Cu E = ? 0.1M 10M Zn2+ 01.0 ]10[ ]1.0[ ][ ][ 2 2 c c Q M M Cu Zn Q 10 M 0.1 M Using Nernst Eqn E0 = Std condition (1M) – 1.10V R = Gas constant (8.31) n = # e transfer (2 e) F = Faraday constant (96 500C mol -1 ) VE E E 16.1 059.010.1 )01.0ln( )965002( )29831.8( 10.1 Non std 0.1/10M E cell increase ↑ [Zn2+] decrease ↓ ↓ Le Chatelier’s principle ↓ Zn2+ + 2e ↔ Zn ↓ [Zn2+] decrease ↓ ↓ Shift to left ← ↓ E → ↑ +ve → Zn more able lose e- E cell increase ↑ [Cu2+] increase ↑ ↓ Le Chatelier’s principle ↓ Cu2+ + 2e ↔ Cu ↓ [Cu2+] increase ↑ ↓ Shift to right → ↓ E→↑ +ve → Cu2+ more able gain e- + [Zn2+] ↓/ [Cu2+] ↑E cell > Eθ Very +ve 1.16 > 1.10
- 12. Eθ value DO NOT depend surface area of metal electrode. E cell = Energy per unit charge. (Joule)/C E cell- 10v = 10J energy released by 1C of charge flowing = 100J energy released by 10C of charge flowing Eθ – intensiveproperty–independentof amt – Ratio energy/charge Increasing surface area metal will NOT increase E cell Eθ Zn/Cu = 1.10V Surface area exposed 10 cm2 Total charges 100C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1C release 1.1 J energy 100 C release 110 J energy Voltmetermeasure energy for 1C – 110J/100C – 1.1V E cell no change Current– measured in Amperes or Coulombs per second 1A = 1 Coulomb charge pass througha point in 1 second = 1C/s 1 Coulomb charge (electron)= 6.28 x 10 18 electronspassing in 1 second 1 electron/protoncarry charge of – 1.6 x 10 -19 C ( very small) 6.28 x 10 18 electron carry charge of - 1 C ond electron ond Coulomb A sec.1 .1028.6 sec1 1 1 18 Surface area increase ↑ Total Energy increase ↑ Total Charge increase ↑Current increase ↑ BUT E cell remainSAME E cell = (Energy/charge)t Q I tIQ Q up ↑ – I up ↑ 100C flow 110J released VEcell Ecell eCh Energy Ecell 10.1 100 110 arg Surfacearea exposed10 cm2 Surfacearea exposed100cm2 Surface area exposed 100 cm2 Total charges 1000C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1 C release 1.1J energy 1000 C release 1100 J energy Voltmetermeasure energy for 1C – 1100J/1000C – 1.1V E cell no change VEcell Ecell eCh Energy Ecell 10.1 1000 1100 arg Eθ Zn/Cu = 1.10V 1000C flow 1100J released t Q I t Q I
- 13. Eθ value DO NOT depend surface area of metal electrode. E cell = Energy per unit charge. (Joule)/C E cell- 10v = 10J energy released by 1C of charge flowing = 100J energy released by 10C of charge flowing Eθ – intensiveproperty–independentof amt – Ratio energy/charge Increasing surface area metal will NOT increase E cell Eθ Zn/Cu = 1.10V Surface area exposed 10 cm2 Total charges 100C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1C release 1.1J energy 100 C release 110 J energy Voltmetermeasure energy for 1C – 110J/100C – 1.1V E cell no change Surface area increase ↑ Total Energy increase ↑ Total Charge increase ↑Current increase ↑ BUT E cell remain SAME E cell = (Energy/charge)t Q I tIQ Q up ↑ – I up ↑ 100C flow 110J released VEcell Ecell eCh Energy Ecell 10.1 100 110 arg Surfacearea exposed10 cm2 Surfacearea exposed100cm2 Surface area exposed 100 cm2 Total charges 1000C leave electrode E cell = 1.1V = 1.1 J energy for 1 C (charges leaving) 1 C release 1.1J energy 1000 C release 1100 J energy Voltmetermeasure energy for 1C – 1100J/1000C – 1.1V E cell no change VEcell Ecell eCh Energy Ecell 10.1 1000 1100 arg Eθ Zn/Cu = 1.10V 1000C flow 1100J released t Q I t Q I Q nF RT EE ln Salt bridge conc Conc of ion E cell depend Nature of electrode Type of metal used Temp of sol T = Temp in K Q = Rxn Quotient E0 = std (1M) n = # e transfer F = Faraday constant (96 500C mol -1 ) R = Gas constant (8.31) Eθ Q T E cell depend Surface area of contact Size of cation/anion
- 14. Eθ value DO NOT depend surface area of metal electrode. E cell = Energy per unit charge. (Joule)/C E cell- 10v = 10J energy released by 1C of charge flowing = 100J energy released by 10C of charge flowing Eθ – intensiveproperty–independentof amt – Ratio energy/charge Increasing surface area metal will NOT increase E cell Surface area increase ↑ Total Energy increase ↑ Total Charge increase ↑Current increase ↑ BUT E cell remain SAME E cell = (Energy/charge) t Q I tIQ Q up ↑ – I up ↑ Q nF RT EE ln Salt bridge conc Conc of ion E cell depend Nature of electrode Type of metal used Temp of sol T = Temp in K Q = Rxn Quotient E0 = std (1M) n = # e transfer F = Faraday constant (96 500C mol -1 ) R = Gas constant (8.31) Eθ Q T E cell depend Salt bridge conc Surface area of contact Size of cation/anion Current/I depend Eθ cell = EMF in V (std condition) Eθ = Show ease/tendency of species to accept/lose electron Eθ = +ve std electrode potential (strong oxidizing agent – weak reducing agent – accept e-) Eθ = - ve std electrode potential (strong reducing agent - weak oxidizing agent – lose e-) Eθ = written as std reduction potential Eθ DO NOT depend on stoichiometric coefficient. EMF = Energy per unit charge. (Joule)/C EMF 10v = 10J energy released by 1C charge = 100J energy released by 10C charge = 1000J energy released by 100C charge Eθ = Intensive property – INDEPENDENT of amt (Ratio energy/charge) Eθ = +ve suggest rxn feasible, does not tell rate (feasible but may be slow, give no indication rate) Eθ = +ve = Energetically feasible but kinetically non feasible Size of cation/anion Surface area of contact Resistance high ↑ – current low ↓ EMF = 10V
