EU Medical Device Classification MDR 2017/745
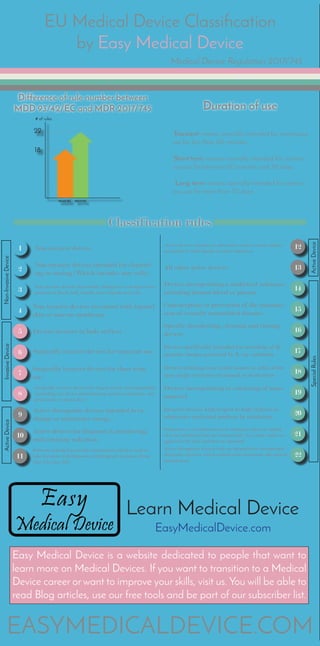
- 1. EU Medical Device Classification by Easy Medical Device Medical Device Regulation 2017/745 22 18 Difference of rule number between MDD 93/42/EC and MDR 2017/745 # of rules 93/42/EC 2017/745 Duration of use ‘Transient’ means normally intended for continuous use for less than 60 minutes. ‘Short term’ means normally intended for continu- ous use for between 60 minutes and 30 days. ‘Long term’ means normally intended for continu- ous use for more than 30 days. Classification rules 9 10 11 12 13 1 2 3 4 5 6 7 8 14 15 16 17 18 19 20 21 22 Non-invasive devices Non-invasive devices intended for channel- ing or storing (Which includes now cells) Non-invasive devices that modify biological or chemical com- position of blood, body liquids, other liquids and cells. Non-invasive devices in contact with injured skin or mucous membrane. Devices invasive in body orifices Surgically invasive devices for transient use. Surgically invasive devices for short term use. Surgically invasive devices for long term use and implantable (including any device administering medicinal products, sur- gical mesh or spinal disc). Active therapeutic devices intended to ex- change or administer energy. Active devices for diagnosis & monitoring, emit ionizing radiation. Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes (from class I to class III) Active devices intended to administer and/or remove medici- nal products, body liquids or other substances All other active devices. Devices incorporating a medicinal substance including human blood or plasma Contraception or prevention of the transmis- sion of sexually transmitted diseases Specific disinfecting, cleaning and rinsing devices Devices specifically intended for recording of di- agnostic images generated by X-ray radiation Devices utilizing non-viable tissues or cells of hu- man origin or tissues of animal or derivatives Devices incorporating or consisting of nano- material Invasive devices with respect to body orifices to administer medicinal products by inhalation Substances or of combinations of substances that are intend- ed to be introduced into the human body via a body orifice or applied to the skin and that are absorbed Active therapeutic devices with an integrated or incorporated diagnostic function which significantly determines the patient management Non-InvasiveDeviceInvasiveDeviceActiveDevice ActiveDeviceSpecialRules Learn Medical Device EasyMedicalDevice.com EASYMEDICALDEVICE.COM Easy Medical Device is a website dedicated to people that want to learn more on Medical Devices. If you want to transition to a Medical Device career or want to improve your skills, visit us. You will be able to read Blog articles, use our free tools and be part of our subscriber list.
