Japan Medical Devices Market
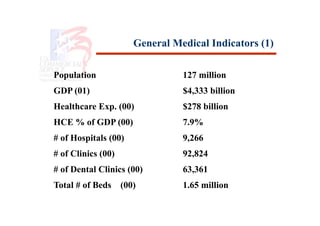
- 1. General Medical Indicators (1) Population 127 million GDP (01) $4,333 billion Healthcare Exp. (00) $278 billion HCE % of GDP (00) 7.9% # of Hospitals (00) 9,266 # of Clinics (00) 92,824 # of Dental Clinics (00) 63,361 Total # of Beds (00) 1.65 million
- 2. General Medical Indicators (2) Total # of Surgeries approx. 5 million Average Hospital Stay (00) 39.1 (24.8 for ordinary hospital) Life Expectation (00) male: 77.64 female: 84.62 Insurance Coverage 100% Concept of Operation not for profit Medical Device Market (00) $18 billion Pharmaceutical Market (00) $60 billion
- 3. Japan’s Healthcare System (1) Major Characteristics Universal Healthcare Coverage (achieved in 1961) - health insurance covering the entire population Free Access - patients can freely choose doctors, clinics, hospitals Low Co-payment Rate - patient can receive necessary medical care for a small fee (patient are required to pay anywhere 10 and 30% of treatment fee) Fee-For-Service - reimbursed based on point system
- 4. Japan’s Healthcare System (2) Rapidly Changing Healthcare Environment Changes in the disease pattern - acute vs. chronic Aging Society and lower birthrate - the elderly will make up a significant percentage of the total population Increasing Healthcare Expenditure - Japan’s spending on medical care continues to increase as Japan becomes a aging society Stagnant Economy - imbalance between the increase of healthcare expenditure and the economic growth will increase further
- 5. Rate of Projected Population - over 65 30 25 Japan Percentage 20 USA France 15 Germany 10 UK Sweden 5 0 2000 2005 2010 2015 2020 2025 Year
- 6. National Healthcare Expenditure Medical Expenditure % Medical Expenditure for the Aged (over 70) 400,000 50 39.1 37.2 100 million yen 34.7 35.7 36.7 37.3 40 Percentage 300,000 28.8 30 200,000 17.5 20 100,000 10 0 0 1980 1990 1995 1996 1997 1998 1999 2000 Year Source: Ministry of Health, Labor and Welfare (MHLW)
- 7. Healthcare Reform? • Past efforts included: - cut reimbursement rates (biannually since 1965) - established a separate insurance system for elderly (1984) - raised co-payment for the elderly (1997) - raised co-payment for ordinary salaried workers (1997) - established a new long-term care insurance system (2000) • Recent efforts (2002) included - cut reimbursement rates * first time cut on doctor’s technical fee * introduction of foreign reference price on medical devices - raised co-payment for the elderly - raised co-payment for ordinary salaried workers
- 8. Japan Medical Device Market $US Bill 20 20 18 18 15 16 14 14 12 12 10 8 5 6 4 2 0 USA Japan UK France Germany Korea
- 9. Medical Device Trade Balances Between U.S. and Japan $US Million CY 1998 CY 1999 CY 2000 Production 11,507 13,051 13,761 Import 6,370 7,319 7,603 - from U.S. 4,045 4,870 4,941 Export 2,499 3,202 3,362 - to U.S. 800 968 949 Total Market 15,379 17,169 18,002 U.S. Trade Surplus 3,245 3,902 3,992 U.S. Share of Import 63.5% 67.0% 65.0% U.S. Share of Market 26.3% 27.0% 27.5% F/X (1 US$/Yen) 131 114 108 Source: Ministry of Health, Labor and Welfare (MHLW)
- 10. Japan Medical Device Imports Medical Device Imports in 2000 United States Germany Netherl Ireland Others 20% 4% 5% 7% 64% Source: Ministry of Health, Labor and Welfare (MHLW)
- 11. Competitive Products (Japan vs U.S.) Japan • Imaging Diagnostic equipment • Therapeutic and Surgical Equipment • Biophenomena Measuring and Monitoring Systems • Home Therapeutic Equipment • Others (Dialyzers, Endoscopes, Hemodialysis Apparatus, etc.) U.S. • Pacemakers • Advanced Interventional Cardiology Products such as stents • Orthopedic Implants • Laser Surgical Equipment • Cardiac Valve Prothesis • Others (MRI, CT, etc.)
- 12. U.S. Medical Device Firms in Japan • 3M Healthcare • General Electric (GE) • Allergan • Guidant • Bausch & Lomb • Johnson & Johnson • Baxter • Kodak • Boston Scientific • Medtronic • Edwards Lifesciences • St. Jude Medical American Chamber of Commerce in Japan (ACCJ), Medial Device Subcommittee has 46 member firms
- 13. Medical Device Regulation System Japanese Law Pharmaceutical Affairs Law (PAL) ----- enforced by the Ministry of Health, Labor and Welfare (MHLW). Necessary Governmental Authorizations • Manufacturing (or import) approval (quot;Shoninquot;) which guarantees the safety and efficacy of the device. (approx. 2,500-3,000 submissions per year) • Manufacturing ( or import) license (quot;Kyokaquot;) of a device, which the Japanese manufacturer and importer hold. (in 2002: manufacturing license - 2,709 / import license - 1,282) • Reimbursement listing approval
- 14. How to Register Your Products Ask Your Importer to Apply Use In-country Care Taker Pros Pros • Simple • Option to work with multiple importers who may have • Less expensive different strenghts • No direct involvement with the • No reapplication for shonin upon Japanese authorities change of importer • The ability to focus fully on marketing your product Cons Cons • The manufacturer often is • Expensive limited to one importer • Change of importer forces the manufacturer to reapply for shonin from the beginning • Dependency on the importer increases
- 15. Application Categories (1) Devices which do not require approval (2) “Me-too Devices” which are equivalent to already- approved devices in Japan (Time Clock: 4 months) (3) “Improved Devices” which do not have new characteristics as much as the devices to be reexamined but are not substantially equivalent to already-approved devices in Japan (Time Clock: 12 months) (4) “New Devices” which are different in use, function or technology from already-approved devices in Japan (Time Clock: 12 months)
- 16. Risk Categories of Medical Devices Categories Categories of medical Approval Clinical Trial devices according to risk Medical devices that are believed to pose extremely little risk to the human body even if Class I they fail ----- Examples: In vitro diagnostic devices, steel supplies, x-ray film, dental prosthetic supplies Not necessary Medical devices that are believed to pose relatively little risk to the human body even if they fail Class II Examples: MRI, electromanometers, In principle, not necessary electronic endoscopes, digestive catheters, ultrasonic diagnostic equipment, and dental alloys Medical devices that are believed to pose relatively high risk to the human body if they Class III fail Sometimes, necessary Examples: dialyzers, artificial bones, Necessary respirators, and balloon catheters Medical devices that are highly invasive upon the patient and may directly endanger the Class IV patient's life if they fail In principle, necessary Examples: pacemakers, artificial heart valves, and stents Note: The products shown as examples are classified according to international categories. Minister of Health, Labor and Welfare has the final authority to classify products according to definitions under the law.
- 17. Submission to Prefecural Government Evaluation and Licensing Div., MHLW Pharmaceuticals and Medical Devices Evaluation Center (PMDEC) New Device Improved Device Me-too Device Evaluation by PMDEC Evaluation by JAAME Presentation Presentation Equivalency Investigation Expert Discussion Expert Discussion Pharmaceuticals and Medical Devices Evaluation Center (PMDEC) Evaluation and Licensing Div., MHLW PAFCS Evaluation and Licensing Div., MHLW Prefecural Government Approval
- 18. Pharmaceutical Affairs Law Revision - Key Points (1) review of the approval system and enhancement of past marketing safety measures * Introduction of a classification system corresponding to the risk of medical devices - three categories by risk to the human body (extremely low, low and high) * A third party certification system for low risk medical devices (2) review of the safety measures regarding medical devices; and (3) enhancement of safety measures for biological products
- 19. Pharmaceutical Affairs Law Revision - Revision Schedule July 2002 Pharmaceutical Affairs Law Amendment (PAL) passed the Diet Fall 2002 Bill on new Administrative Agency to be submitted to extraordinary session of the Diet April 2003 Revised PAL on biological products to be enacted April 2004 New Independent Administrative Corporation to be established April 2005 Full enforcement of revised PAL
- 20. Review of Categories and Safety Measures Concerning Medical Devices Current Pharmaceutical Revision plan Current status and Affairs Law International review plan Distribution Distribution categories Categories of medical regulations regulations devices according to risk Risk Primary Manufacturing distribution regulations regulations Distributor's notification Extremely low Notification for is not required Medical devices that are believed to distribution Class I pose extremely little risk to the human is not necessary Approval for body even if they fail marketing authorization is not Approval of required manufacturing is Distributor's Notification not necessary is required* Medical devices that are believed to Imple- Class II pose relatively little risk to the human Low mentation body even if they fail of third-party certification Notification system system for distribution Medical devices that are believed to Implementation pose relatively high risk to the human of license Class III body if they fail system for distribution Medical devices that are highly Minister's High invasive upon the patient and may approval for Class IV directly endanger the patient's life if manufacturing Minister's they fail approval for marketing authorization
- 21. New Independent Administrative Agency Current System Proposed New System Drug Medical Device Drug and Medical Device Manufacturers Manufacturers Manufacturers Inquiry, instruction Inquiry, instruction Inquiry, instruction Application Application and answer and answer and answer OPSR PMDEC JAAME New Independent Drug’s equivalency Administrative Institution investigation. Clinical trial Review of drugs Medical devices’ consultation. and medical equivalency Review of drugs and medical Reliability devices investigation devices (including clinical trial inspections. GCP, consultation and inspections) GLP and GPMSP inspections Report of review results Report of review results Consultation Consultation MHLW Council MHLW Council Recommendation Recommendation Approval Approval Certification by a third party for low-risk medical devices
- 22. Medical Device Reimbursement A1 Covered under technical fee: Product reimbursement is included in the technical fee. Products are usually a low-end, inexpensive nature such as sutures and other supplies. A2 Covered under technical fee: Product itself gives technical fees. A2 products include high-end and expensive diagnostic equipment such as MRI, CT, etc. B Existing products (single use products): There is an existing technical fee and also existing device category. C1 New products (single use products) used with current existing technology: There is an existing technical fee, but no existing device category. C2 New products with no existing technology: This category is for breakthrough technologies.
- 23. Seriously, Japan is a tough market • Cost contamination pressure • Over-regulation • High-costs of doing business • Protectionism • Unique and complex market culture • Competitive Japanese firms
- 24. Approaching the Market • Trade Shows - Modern Hospital Show - Hospex - Japan Dental Show - and more • Academic Conference and Exhibition • Healthcare Consultants • The U.S. Commercial Service Programs
- 25. Market Information in English • Our market research reports http://www.usatrade.gov • Japan External Trade Organization's (JETRO) http://www.jetro.go.jp • Japan Pharmaceutical Manufacturers Association (JPMA) http://www.jpma.or.jp • Ministry of Health, Labor and Welfare (MHLW) http://www.mhlw.go.jp/english/index.html • Japan Association for the Advancement of Medical Equipment (JAAME) http://www.jaame.or.jp/english/index.html