IEC 62304 Action List
•
8 likes•5,267 views
IEC 62304 is an international standard that defines software development lifecycle requirements for medical device software. It requires that all aspects of the software development life cycle be scrutinized to ensure patient safety when software is involved. The standard establishes software safety classes A, B, and C based on the possible risk to health from software failures. It also outlines numerous requirements for each class, including developing plans, requirements, designs, testing procedures, problem resolution processes, and more. Upon completion, all documentation should be submitted to a test lab for review to obtain certification.
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
[Note: This is a partial preview. To download this presentation, visit:
https://www.oeconsulting.com.sg/training-presentations]
ISO 13485:2016 is an international standard that sets out the requirements for a quality management system (QMS) specific to the medical devices industry. The standard focuses on meeting customer and applicable regulatory requirements and is intended for any organization partially or fully involved in the medical device life-cycle.
This presentation can be used to brief your employees, new hires and potential auditees so as to create awareness of the ISO 13485:2016 standard. Alternatively, the presentation may be used to supplement your materials for the training of QA professionals and internal auditors in the medical devices industry.
It covers the what and why of ISO 13485, the QMS key clause structure, the audit approach and also offers practical tips on how to handle an audit session. When you are done teaching this material to your employees, they will be much more informed and comfortable with ISO 13485:2016.
LEARNING OBJECTIVES
1. Provide background knowledge on ISO 13485:2016
2. Gain an overview of ISO 13485:2016 structure and the certification process
3. Understand the audit approach
4. Gather useful tips on handling an audit session
CONTENTS
1. Overview of ISO 13485
About ISO
What are Standards?
Why are Standards Important?
What is ISO 13485?
Who is ISO 13485 For?
What is a Medical Device?
What is a Quality Management System?
How Does ISO 13485 Work?
Benefits that ISO 13485 Will Bring to the Organization
Advantages of Certification
Development of ISO 13485
Why Was ISO 13485 Revised?
Key Improvements to ISO 13485:2016
Relationship of ISO 13485 with ISO 9001
2. ISO 13485:2016 Structure
The ISO 13485:2016 Structure
The Plan-Do-Check-Act (PDCA) Process Model
ISO 13485:2016 Approach is Based on the PDCA Cycle
Documentation Requirements
ISO 13485:2016 Key Clause Structure (4-8)
Clause 4: Quality Management System
Clause 5: Management Responsibility
Clause 6: Resource Management
Clause 7: Product Realization
Clause 8: Measurement, Analysis & Improvement
3. ISO 13485:2016 Certification
Becoming ISO 13485:2016 Certified
ISO 13485:2016 Certification Process
4. Audit Approach
What is a Quality Audit?
What Are Audits Used For?
Types of Quality Audits
Internal Quality Audit
Principles of Auditing
Audit Focus
Audit Approach
Audit Emphasis
Document Review
Audit Findings
5. Handling an Audit Session
Rights of Auditee
Rights of Auditor
How to Handle an Audit Session?
Auditee's Conduct
Interacting with Auditors: Do's
Interacting with Auditors: Don'tsISO 13485:2016 (Medical Devices - Quality Management System) Awareness Training

ISO 13485:2016 (Medical Devices - Quality Management System) Awareness TrainingOperational Excellence Consulting
Check out our latest webinar to learn more about complying with IEC 62304, ISO 14971, IEC 60601, and relevant FDA regulations (for instance, Title 21 CFR Part 11 about electronic signatures). In this webinar, we discussed the requirements set forth by these standards. We also showed our Intland's Medical IEC 62304 Template to leverage codeBeamer ALM's advanced capabilities and to facilitate compliance with these regulations.Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...

Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...Intland Software GmbH
More Related Content
What's hot
[Note: This is a partial preview. To download this presentation, visit:
https://www.oeconsulting.com.sg/training-presentations]
ISO 13485:2016 is an international standard that sets out the requirements for a quality management system (QMS) specific to the medical devices industry. The standard focuses on meeting customer and applicable regulatory requirements and is intended for any organization partially or fully involved in the medical device life-cycle.
This presentation can be used to brief your employees, new hires and potential auditees so as to create awareness of the ISO 13485:2016 standard. Alternatively, the presentation may be used to supplement your materials for the training of QA professionals and internal auditors in the medical devices industry.
It covers the what and why of ISO 13485, the QMS key clause structure, the audit approach and also offers practical tips on how to handle an audit session. When you are done teaching this material to your employees, they will be much more informed and comfortable with ISO 13485:2016.
LEARNING OBJECTIVES
1. Provide background knowledge on ISO 13485:2016
2. Gain an overview of ISO 13485:2016 structure and the certification process
3. Understand the audit approach
4. Gather useful tips on handling an audit session
CONTENTS
1. Overview of ISO 13485
About ISO
What are Standards?
Why are Standards Important?
What is ISO 13485?
Who is ISO 13485 For?
What is a Medical Device?
What is a Quality Management System?
How Does ISO 13485 Work?
Benefits that ISO 13485 Will Bring to the Organization
Advantages of Certification
Development of ISO 13485
Why Was ISO 13485 Revised?
Key Improvements to ISO 13485:2016
Relationship of ISO 13485 with ISO 9001
2. ISO 13485:2016 Structure
The ISO 13485:2016 Structure
The Plan-Do-Check-Act (PDCA) Process Model
ISO 13485:2016 Approach is Based on the PDCA Cycle
Documentation Requirements
ISO 13485:2016 Key Clause Structure (4-8)
Clause 4: Quality Management System
Clause 5: Management Responsibility
Clause 6: Resource Management
Clause 7: Product Realization
Clause 8: Measurement, Analysis & Improvement
3. ISO 13485:2016 Certification
Becoming ISO 13485:2016 Certified
ISO 13485:2016 Certification Process
4. Audit Approach
What is a Quality Audit?
What Are Audits Used For?
Types of Quality Audits
Internal Quality Audit
Principles of Auditing
Audit Focus
Audit Approach
Audit Emphasis
Document Review
Audit Findings
5. Handling an Audit Session
Rights of Auditee
Rights of Auditor
How to Handle an Audit Session?
Auditee's Conduct
Interacting with Auditors: Do's
Interacting with Auditors: Don'tsISO 13485:2016 (Medical Devices - Quality Management System) Awareness Training

ISO 13485:2016 (Medical Devices - Quality Management System) Awareness TrainingOperational Excellence Consulting
What's hot (20)
Software as a Medical Device (SaMD) Challenges and Opportunities for 2021 and...

Software as a Medical Device (SaMD) Challenges and Opportunities for 2021 and...
Difference between fda 21 cfr part 820 and ISO 13485

Difference between fda 21 cfr part 820 and ISO 13485
FDA Expectations for Traceability in Device & Diagnostic Design

FDA Expectations for Traceability in Device & Diagnostic Design
Quality Control for Medical Device Software - It Arena Lviv Presentation

Quality Control for Medical Device Software - It Arena Lviv Presentation
Excel spreadsheets how to ensure 21 cfr part 11 compliance

Excel spreadsheets how to ensure 21 cfr part 11 compliance
Computerized System Validation : Understanding basics 

Computerized System Validation : Understanding basics
ISO 14971:2019 and ISO/TR 24971 Risk Management Update

ISO 14971:2019 and ISO/TR 24971 Risk Management Update
ISO 13485:2016 (Medical Devices - Quality Management System) Awareness Training

ISO 13485:2016 (Medical Devices - Quality Management System) Awareness Training
Medical Devices Regulation (MDR) 2017/745 - Postmarket surveillance 

Medical Devices Regulation (MDR) 2017/745 - Postmarket surveillance
ISO: 14971 Quality risk management of medical devices

ISO: 14971 Quality risk management of medical devices
Viewers also liked
Check out our latest webinar to learn more about complying with IEC 62304, ISO 14971, IEC 60601, and relevant FDA regulations (for instance, Title 21 CFR Part 11 about electronic signatures). In this webinar, we discussed the requirements set forth by these standards. We also showed our Intland's Medical IEC 62304 Template to leverage codeBeamer ALM's advanced capabilities and to facilitate compliance with these regulations.Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...

Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...Intland Software GmbH
Viewers also liked (12)
Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...

Compliance with medical standards iec 62304, iso 14971, iec 60601, fda title ...
Death by documentation - Medical Device Development Challenges

Death by documentation - Medical Device Development Challenges
Human factor standards and usability (by Ed Israelski)

Human factor standards and usability (by Ed Israelski)
QAdvis - software risk management based on IEC/ISO 62304

QAdvis - software risk management based on IEC/ISO 62304
PECB Webinar: Overview of ISO 13485 - Medical Devices

PECB Webinar: Overview of ISO 13485 - Medical Devices
User Interface Design for Medical Devices - The Relationship Between Usabilit...

User Interface Design for Medical Devices - The Relationship Between Usabilit...
Similar to IEC 62304 Action List
This presentation reviews the regulatory requirements for intended use validation of SaaS-based EDC systems from the Sponsor and CRO perspective and provides best practices for implementing the proper validation in your organization.Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs

Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROsStatistics & Data Corporation
Similar to IEC 62304 Action List (20)
Process and Regulated Processes Software Validation Elements

Process and Regulated Processes Software Validation Elements
Verification and Validation in Software Engineering SE19

Verification and Validation in Software Engineering SE19
Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs

Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs
Recently uploaded
Falcon stands out as a top-tier P2P Invoice Discounting platform in India, bridging esteemed blue-chip companies and eager investors. Our goal is to transform the investment landscape in India by establishing a comprehensive destination for borrowers and investors with diverse profiles and needs, all while minimizing risk. What sets Falcon apart is the elimination of intermediaries such as commercial banks and depository institutions, allowing investors to enjoy higher yields.Falcon Invoice Discounting: The best investment platform in india for investors

Falcon Invoice Discounting: The best investment platform in india for investorsFalcon Invoice Discounting
Saudi Arabia [ Abortion pills) Jeddah/riaydh/dammam/++918133066128☎️] cytotec tablets uses abortion pills 💊💊 How effective is the abortion pill? 💊💊 +918133066128) "Abortion pills in Jeddah" how to get cytotec tablets in Riyadh " Abortion pills in dammam*💊💊 The abortion pill is very effective. If you’re taking mifepristone and misoprostol, it depends on how far along the pregnancy is, and how many doses of medicine you take:💊💊 +918133066128) how to buy cytotec pills
At 8 weeks pregnant or less, it works about 94-98% of the time. +918133066128[ 💊💊💊 At 8-9 weeks pregnant, it works about 94-96% of the time. +918133066128) At 9-10 weeks pregnant, it works about 91-93% of the time. +918133066128)💊💊 If you take an extra dose of misoprostol, it works about 99% of the time. At 10-11 weeks pregnant, it works about 87% of the time. +918133066128) If you take an extra dose of misoprostol, it works about 98% of the time. In general, taking both mifepristone and+918133066128 misoprostol works a bit better than taking misoprostol only. +918133066128 Taking misoprostol alone works to end the+918133066128 pregnancy about 85-95% of the time — depending on how far along the+918133066128 pregnancy is and how you take the medicine. +918133066128 The abortion pill usually works, but if it doesn’t, you can take more medicine or have an in-clinic abortion. +918133066128 When can I take the abortion pill?+918133066128 In general, you can have a medication abortion up to 77 days (11 weeks)+918133066128 after the first day of your last period. If it’s been 78 days or more since the first day of your last+918133066128 period, you can have an in-clinic abortion to end your pregnancy.+918133066128
Why do people choose the abortion pill? Which kind of abortion you choose all depends on your personal+918133066128 preference and situation. With+918133066128 medication+918133066128 abortion, some people like that you don’t need to have a procedure in a doctor’s office. You can have your medication abortion on your own+918133066128 schedule, at home or in another comfortable place that you choose.+918133066128 You get to decide who you want to be with during your abortion, or you can go it alone. Because+918133066128 medication abortion is similar to a miscarriage, many people feel like it’s more “natural” and less invasive. And some+918133066128 people may not have an in-clinic abortion provider close by, so abortion pills are more available to+918133066128 them. +918133066128 Your doctor, nurse, or health center staff can help you decide which kind of abortion is best for you. +918133066128 More questions from patients: Saudi Arabia+918133066128 CYTOTEC Misoprostol Tablets. Misoprostol is a medication that can prevent stomach ulcers if you also take NSAID medications. It reduces the amount of acid in your stomach, which protects your stomach lining. The brand name of this medication is Cytotec®.+918133066128) Unwanted Kit Mifty kit IN Salmiya (+918133066128) Abortion pills IN Salmiyah Cytotec pills

Mifty kit IN Salmiya (+918133066128) Abortion pills IN Salmiyah Cytotec pillsAbortion pills in Kuwait Cytotec pills in Kuwait
Falcon stands out as a top-tier P2P Invoice Discounting platform in India, bridging esteemed blue-chip companies and eager investors. Our goal is to transform the investment landscape in India by establishing a comprehensive destination for borrowers and investors with diverse profiles and needs, all while minimizing risk. What sets Falcon apart is the elimination of intermediaries such as commercial banks and depository institutions, allowing investors to enjoy higher yields.Unveiling Falcon Invoice Discounting: Leading the Way as India's Premier Bill...

Unveiling Falcon Invoice Discounting: Leading the Way as India's Premier Bill...Falcon Invoice Discounting
Recently uploaded (20)
Marel Q1 2024 Investor Presentation from May 8, 2024

Marel Q1 2024 Investor Presentation from May 8, 2024
Falcon Invoice Discounting: The best investment platform in india for investors

Falcon Invoice Discounting: The best investment platform in india for investors
Lucknow Housewife Escorts by Sexy Bhabhi Service 8250092165

Lucknow Housewife Escorts by Sexy Bhabhi Service 8250092165
TVB_The Vietnam Believer Newsletter_May 6th, 2024_ENVol. 006.pdf

TVB_The Vietnam Believer Newsletter_May 6th, 2024_ENVol. 006.pdf
Uneak White's Personal Brand Exploration Presentation

Uneak White's Personal Brand Exploration Presentation
Mifty kit IN Salmiya (+918133066128) Abortion pills IN Salmiyah Cytotec pills

Mifty kit IN Salmiya (+918133066128) Abortion pills IN Salmiyah Cytotec pills
Falcon Invoice Discounting: Tailored Financial Wings

Falcon Invoice Discounting: Tailored Financial Wings
Unveiling Falcon Invoice Discounting: Leading the Way as India's Premier Bill...

Unveiling Falcon Invoice Discounting: Leading the Way as India's Premier Bill...
Escorts in Nungambakkam Phone 8250092165 Enjoy 24/7 Escort Service Enjoy Your...

Escorts in Nungambakkam Phone 8250092165 Enjoy 24/7 Escort Service Enjoy Your...
Paradip CALL GIRL❤7091819311❤CALL GIRLS IN ESCORT SERVICE WE ARE PROVIDING

Paradip CALL GIRL❤7091819311❤CALL GIRLS IN ESCORT SERVICE WE ARE PROVIDING
HomeRoots Pitch Deck | Investor Insights | April 2024

HomeRoots Pitch Deck | Investor Insights | April 2024
Quick Doctor In Kuwait +2773`7758`557 Kuwait Doha Qatar Dubai Abu Dhabi Sharj...

Quick Doctor In Kuwait +2773`7758`557 Kuwait Doha Qatar Dubai Abu Dhabi Sharj...
Falcon Invoice Discounting: Unlock Your Business Potential

Falcon Invoice Discounting: Unlock Your Business Potential
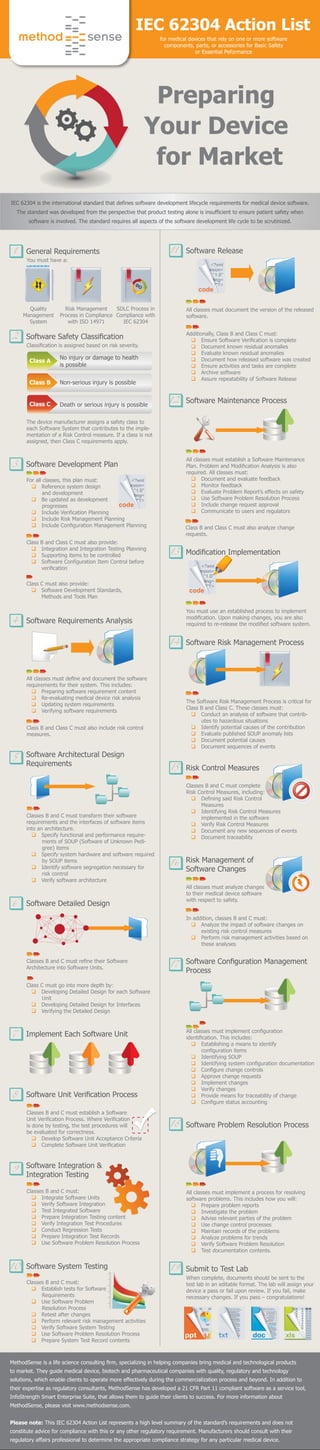
IEC 62304 Action List
- 1. You must have a: Quality Management System IEC 62304 Action List for medical devices that rely on one or more software components, parts, or accessories for Basic Safety or Essential Peformance General Requirements1 Classification is assigned based on risk severity. The device manufacturer assigns a safety class to each Software System that contributes to the imple- mentation of a Risk Control measure. If a class is not assigned, then Class C requirements apply. Software Safety Classification2 For all classes, this plan must: q Reference system design and development q Be updated as development progresses q Include Verification Planning q Include Risk Management Planning q Include Configuration Management Planning Class B and Class C must also provide: q Integration and Integration Testing Planning q Supporting items to be controlled q Software Configuration Item Control before verification Software Development Plan3 Class A No injury or damage to health is possible Class B Non-serious injury is possible Class C All classes must define and document the software requirements for their system. This includes: q Preparing software requirement content q Re-evaluating medical device risk analysis q Updating system requirements q Verifying software requirements Software Requirements Analysis4 Classes B and C must transform their software requirements and the interfaces of software items into an architecture. q Specify functional and performance require- ments of SOUP (Software of Unknown Pedi- gree) items q Specify system hardware and software required by SOUP items q Identify software segregation necessary for risk control q Verify software architecture Software Architectural Design Requirements 5 Class C must also provide: q Software Development Standards, Methods and Tools Plan Class B and Class C must also analyze change requests. Class B and Class C must also include risk control measures. Class C must go into more depth by: q Developing Detailed Design for each Software Unit q Developing Detailed Design for Interfaces q Verifying the Detailed Design Software Detailed Design6 Implement Each Software Unit7 Software Unit Verification Process8 Classes B and C must refine their Software Architecture into Software Units. Classes B and C must establish a Software Unit Verification Process. Where Verification is done by testing, the test procedures will be evaluated for correctness. q Develop Software Unit Acceptance Criteria q Complete Software Unit Verification Software Integration & Integration Testing 9 Classes B and C must: q Integrate Software Units q Verify Software Integration q Test Integrated Software q Prepare Integration Testing content q Verify Integration Test Procedures q Conduct Regression Tests q Prepare Integration Test Records q Use Software Problem Resolution Process Death or serious injury is possible Risk Management Process in Compliance with ISO 14971 SDLC Process in Compliance with IEC 62304 code Additionally, Class B and Class C must: q Ensure Software Verification is complete q Document known residual anomalies q Evaluate known residual anomalies q Document how released software was created q Ensure activities and tasks are complete q Archive software q Assure repeatability of Software Release All classes must document the version of the released software. Software Release11 All classes must establish a Software Maintenance Plan. Problem and Modification Analysis is also required. All classes must: q Document and evaluate feedback q Monitor feedback q Evaluate Problem Report’s effects on safety q Use Software Problem Resolution Process q Include change request approval q Communicate to users and regulators Software Maintenance Process12 You must use an established process to implement modification. Upon making changes, you are also required to re-release the modified software system. Modification Implementation13 All classes must analyze changes to their medical device software with respect to safety. When complete, documents should be sent to the test lab in an editable format. The lab will assign your device a pass or fail upon review. If you fail, make necessary changes. If you pass – congratulations! Risk Management of Software Changes 16 All classes must implement configuration identification. This includes: q Establishing a means to identify configuration items q Identifying SOUP q Identifying system configuration documentation q Configure change controls q Approve change requests q Implement changes q Verify changes q Provide means for traceability of change q Configure status accounting Software Configuration Management Process 17 All classes must implement a process for resolving software problems. This includes how you will: q Prepare problem reports q Investigate the problem q Advise relevant parties of the problem q Use change control processes q Maintain records of the problems q Analyze problems for trends q Verify Software Problem Resolution q Test documentation contents. Software Problem Resolution Process18 Submit to Test Lab19 The Software Risk Management Process is critical for Class B and Class C. These classes must: q Conduct an analysis of software that contrib- utes to hazardous situations q Identify potential causes of the contribution q Evaluate published SOUP anomaly lists q Document potential causes q Document sequences of events Software Risk Management Process14 Classes B and C must complete Risk Control Measures, including: q Defining said Risk Control Measures q Identifying Risk Control Measures implemented in the software q Verify Risk Control Measures q Document any new sequences of events q Document traceability Risk Control Measures15 In addition, classes B and C must: q Analyze the impact of software changes on existing risk control measures q Perform risk management activities based on these analyses Software System Testing10 Classes B and C must: q Establish tests for Software Requirements q Use Software Problem Resolution Process q Retest after changes q Perform relevant risk management activities q Verify Software System Testing q Use Software Problem Resolution Process q Prepare System Test Record contents code code IEC 62304 is the international standard that defines software development lifecycle requirements for medical device software. The standard was developed from the perspective that product testing alone is insufficient to ensure patient safety when software is involved. The standard requires all aspects of the software development life cycle to be scrutinized. Preparing Your Device for Market MethodSense is a life science consulting firm, specializing in helping companies bring medical and technological products to market. They guide medical device, biotech and pharmaceutical companies with quality, regulatory and technology solutions, which enable clients to operate more effectively during the commercialization process and beyond. In addition to their expertise as regulatory consultants, MethodSense has developed a 21 CFR Part 11 compliant software as a service tool, InfoStrength Smart Enterprise Suite, that allows them to guide their clients to success. For more information about MethodSense, please visit www.methodsense.com. Please note: This IEC 62304 Action List represents a high level summary of the standard’s requirements and does not constitute advice for compliance with this or any other regulatory requirement. Manufacturers should consult with their regulatory affairs professional to determine the appropriate compliance strategy for any particular medical device.
