Thesis defense
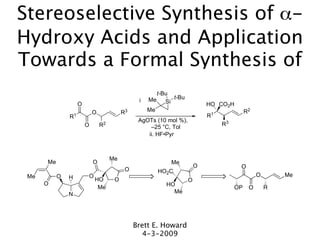
- 1. Stereoselective Synthesis of α- Hydroxy Acids and Application Towards a Formal Synthesis of t-Bu Me t-Bu i Si O HO CO2H O R3 Me R2 R1 R1 AgOTs (10 mol %), O R2 R3 –25 °C, Tol ii. HF•Pyr Me Me O Me O O O HO2C Me O H O O Me HO O O O HO Me OP O R N Me Brett E. Howard 4-3-2009
- 2. Background: Silylene Transfer t-Bu O Si t-Bu t-Bu t-Bu Si t-Bu H Ph O n-Bu n-Bu t-Bu Si Ph AgOCOCF3 ZnBr n-Bu (5 mol %) (20 mol %) 100% 73% d.r. 65:35 • Metal-catalyzed silylene transfer provides silacyclopropanes t-Bu OH OH t-Bu Si O t-BuOOH, CsOH•H2O, Ph Bu4NF, DMF, 70 °C Me Ph Me Me Me 64% Single Diastereomer • Silicon-carbon bonds can be oxidized using modified Tamao conditions Smitrovich, J. H.; Woerpel, K. A. J. Org. Chem. 1996, 61, 6044-6046. Ćiraković, J.; Driver, T. G.; Woerpel, K. A. J. Org. Chem. 2004, 69, 4007-4012.
- 3. Silylene Transfer To Esters t-Bu 1. Si t-Bu t-Bu O O AgOTf (5 mol %) t-Bu Si O t-Bu O t-Bu 2. TMEDA 67% (1H NMR) • Non-enolizable esters give silacyclopropanation products t-Bu t-Bu O Si t-Bu Si OH t-Bu O i-Pr O AgOTf (1 mol%) 45% t-Bu t-Bu t-Bu O Si O Si t-Bu Me EtO EtO AgOCOCF3 (5 mol%) 98% (1H NMR) Ćiraković, J.; Driver, T. G.; Woerpel, K. A. J. Am. Chem. Soc. 2002, 124, 9370-9371. Calad, A. S.; Woerpel, K. A. J. Am. Chem. Soc. 2005, 127, 2046-2047.
- 4. Proposed Silacarbonyl Ylide t-Bu t-Bu t-Bu t-Bu TfO Si Si t-Bu O O O [AgLn] Si t-Bu O i-Pr O i-Pr O i-Pr t-Bu t-Bu t-Bu t-Bu Si OH Si O H O O Me Me t-Bu t-Bu t-Bu t-Bu O Si Si t-Bu t-Bu O O Si EtO Me AgOCOCF3 EtO EtO (5 mol%) Driver, T. G.; Woerpel, K. A. J. Am. Chem. Soc. 2004, 126, 9993-10002.
- 5. Silacarbonyl Ylide Formation Me Me Me Me hv (> 460nm) Me Me hv (254nm) or ∆ SiMes2 Mes2Si + O O SiMes2 hv (254nm) O Me Me Me Me Me Me 62% R2 O SiR2 Si pyrolysis or hv O O O H R2Si + or SiR2 R' Me R' Me R' Me R' 24-62% O t-Bu t-Bu t-Bu t-Bu Si Si HF•Pyr H Ph O O HO OH Me Me CuI (10 mol%) Ph Ph Ph Ph 61%, 2 Steps d.r. 93:7 Ando, W.; Haglwara,K.; Sekiguchi, A. Organometallics 1987, 6, 2270-2271. Ando, W.; Ikeno, M.; Sekiguchi, A. J. Am. Chem. Soc. 1977, 99, 6447-6449. Franz, A.K.; Woerpel, K. A. J. Am. Chem. Soc. 1999, 121, 949-957.
- 6. Proposed α-Keto Ester t-Bu t-Bu O Si Si t-Bu 6π e- O t-Bu O O O Me AgOCOCF3 O (1 mol%) Me t-Bu t-Bu t-Bu t-Bu O Si t-Bu t-Bu Si O O Si O [3,3] O = O O Me O O Me Me •Ireland–Claisen expected to occur through chair TS
- 7. Silylene Transfer to 1,2 Me O OMe MeO O Si + Ph O O Si Me Ph Ph Ph 24% •Addition to 1,2 dicarbonyls has been observed previously t-Bu t-Bu t-Bu O 1 eqv. Si t-Bu Si OEt O O Me O C6D6, AgOCOCF3 Me OEt (1 mol%), 15 min, rt 29Si NMR δ 14.2 ppm Heinicke, J.; Gehrhus, B. J. Organomet. Chem. 1992, 423, 13-21.
- 8. Silylene Transfer to α-Keto Esters O O DCC, DMAP, OH + O Ph HO Ph CH2Cl2, 0 °C O O 58% t-Bu t-Bu t-Bu t-Bu t-Bu 1 eqv. Si Si O Si O t-Bu O O O Ph O Ph Ph C6D6, AgOCOCF3 O O O (1 mol%), 15 min, rt 52% 1H NMR 29Si NMR δ 15.2 ppm •Intermediate dioxacyclopentene not observed
- 9. Silylene Transfer Optimization t-Bu t-Bu O O Si conditions O O Ph Ph O O Entry Catalyst mol % Silylene Source Silylene eqv Conditions % Yield (1H NMR) 1 AgOCOCF3 1 cyclohexyl 1 15 min, rt 53 2 AgOCOCF3 1 dimethyl 1 15 min, rt 61 3 Ag3PO4 10 cyclohexyl 1 2 h, 50 °C 46 4 Ag3PO4 10 dimethyl 1 20 h 62 5 AgOTs 10 cyclohexyl 1 15 min, rt 63 6 AgOTs 10 dimethyl 1 15 min, rt 73 7 AgOBz 10 cyclohexyl 1 15 min, rt 53 8 AgOBz 10 dimethyl 1 15 min, rt 72 9 Cu(OTf)2 1 cyclohexyl 1 2 h, 50 °C 27 10 AgOTs 10 cyclohexyl 1.3 15 min, rt 64 11 AgOTs 10 cyclohexyl 1.6 15 min, rt 73 12 AgOTs 10 dimethyl 1.6 15 min, rt 80 t-Bu t-Bu t-Bu dimethylsilacyclopropane Me Si Si cyclohexylsilacyclopropane t-Bu Me
- 10. Transfer Substrate Scope t-Bu Me t-Bu Si Me O i. AgOTs (10 mol %), HO CO2H –25 °C O R2 R1 R1 O ii. HF•Pyr, rt R2 ≥ 97:3 Entry R1 R2 % Yield 1 Me Ph 70 2 Et Ph 84 3 i-Pr Ph 54 4 t-Bu Ph 47 5 Ph Ph 71 6 Ph Me 62 7 Ph n-Bu 72 8 Ph CH2OTBS 71 9 Et (CH2)2OBn 75
- 11. Chiral α-Hydroxy Acid Synthesis i. (COCl)2, DMF, O O Me Me CH2Cl2 Me + Me OH O n-Bu HO n-Bu ii. Pyr OTBDPS OTBDPS 40% > 97% ee O Me 1. TBAF, THF, –20 °C Me O n-Bu 2. DMP, Pyr, CH2Cl2 O 62% yield, 2 steps
- 12. Chiral α-Hydroxy Acid Synthesis t-Bu Me t-Bu Si t-Bu Me Si t-Bu O O O 4 n-Bu 10 mol % AgOTs 1 O 6π electrocyclization Me 1 R 1 6 O Me O 4 n-Bu 6 Me t-Bu t-Bu t-Bu t-Bu Si O Si [3,3] O HO CO2H O HF•Pyr O Me n-Bu Me O Me 6 O 4 n-Bu n-Bu Me Me Me 71%, >98% ee
- 13. Confirmation of Stereochemistry NH3 Ph Me HO CO2 Me Me n-Bu
- 14. Chiral α-Hydroxy Acid Synthesis NH2 NaNO2, AcOH, OH i. TBSCl, DMF, imid OTBS Me Me Me CO2H CO2H ii. KOH, MeOH, H O CO2H H2O 2 OBn OBn OBn 71% 97% Cl O Cl Cl Cl OTBS 1. TBAF, THF, –20 °C O Me O Ph Me O Ph cinnamyl alcohol, 2. DMP, Pyr, CH2Cl2 DMAP,NEt3, OBn O OBn O PhH 54% 56%, > 98% ee
- 15. Chiral α-Hydroxy Acid Synthesis t-Bu Me t-Bu t-Bu t-Bu Si Me Si O O HO CO2H O 10 mol % AgOTs Me Me O 4 Ph 6 Ph 1 O 4 6 OBn O OBn Ph Me OBn H 63%, 80% diastereoselectivity
- 16. Silylene Transfer to Imines t-Bu t-Bu O Si Si t-Bu 6π e- NR t-Bu O O NR AgOCOCF3 O (1 mol%) t-Bu t-Bu t-Bu t-Bu O Si t-Bu t-Bu Si O O Si N R [3,3] O = R N N R O O
- 17. Silylene Transfer to Imines OH Cl Ph O H5IO6, THF O HO2C OH CO2H Ph O Ph O OH NEt3, DMF OH OH 2 100%, complex 61% mixture of oligomers NH2Bn, CH2Cl2 O MgSO4 Ph O NBn H2N O OMe Ph O CH2Cl2, MgSO4 NPMP
- 18. Silylene Transfer to Imines t-Bu Me t-Bu Si Me O AgOTs (10 mol %), Ph O Decomp. NBn –25 °C, Tol t-Bu Me t-Bu Si t-Bu Me t-Bu O PMPN Si AgOTs (10 mol %), O Ph O NPMP –25 °C, Tol Ph O 48% • p-methoxyphenyl group provided transfer product
- 19. Methodology Limits t-Bu Me t-Bu Si Me i. AgOTs (10 mol %), O O –25 °C HO CO2H O Ph Ph ii. HF•Pyr, rt O O Me HO CO2H Me O O O Ph O Ph O O O Me Me O HO CO2H O Me Ph Ph O Me Me Me
- 20. (+)-Latifoline Me Me O O Me O H O HO O O Me N • Anti leukemia and solid tumor activity • Activity arises from DNA-Protein or DNA interstrand crosslinking • Synthesized by Wood in 2001 Crowley, H. C.; Culvenor, C. C. J. Aust. J. Chem. 1962, 15, 139–144. Rajski, S. R.; Williams, R. M. Chem. Rev. 1998, 98, 2723–2795.
- 21. (+)-Latifoline Retrosynthesis Me Me O O Me O H O HO O O Me N (+)-latifoline Me Me HO OH O H HO2C Me + + O OH HO O N Me angelic acid retronecine (+)-latifolic acid
- 22. (+)-Latifoline Retrosynthesis O O R Me Me O Me O OH O Me HO2C HO2C OP HO2C HO Me HO OP O R Me HO Me OP Me OH + HO Me OP O R O Me R
- 23. α-Keto Ester Synthesis Cl O Cl OTBS OTBS OH Cl Cl Me + Me O Me CO2H Me Et DMAP,NEt3, PhH OBn OBn O Et 86% O 1. TBAF, THF –20 °C Me O Me 2. DMP, Pyr, CH2Cl2 OBn O Et 71%, 2 steps
- 24. Silylene Transfer t-Bu Me t-Bu Si Me O i. AgOTs (10 mol %), HO2C OH Me O Me –25 °C Me Et OBn O Et ii. HF•Pyr, rt OBn Me 50:50 dr, 72%
- 25. Transition State Analysis t-Bu t-Bu Si O R R R 4 120° 4 120° 4 6 O Et Me rotation Et Me rotation Et Me Me O 1 R O R O R O 4 1 Et 1 Me OBn H Me BnO H BnO H H Me Me BnO σC–Me σ∗C–C R = dioxosilacyclopentyl 4 Et Me HO2C OH HO2C OH Me Et 1 BnO OBn Me H Me
- 26. Transition State Analysis t-Bu t-Bu Et Si Et O R HO CO2H Et O 4 4 Me Me Me 6 Me R O HO CO2H O 4 1 1 OBn Me Et Me OBn BnO H BnO H H Me Me σC–Me σ∗C–C R = dioxosilacyclopentyl Et A value 1.75 Me A Value 1.70
- 27. Transition State Analysis CF3 O CF3 OTMS CF3 O i. LDA PdCl2(PhCN)2 1 i-Pr O i-Pr O i-Pr OH ii. TMSCl reflux 6 4 6 4 74% yield, 88% syn O CH3 O O CH3 OTMS O CH3 O i. LDA PdCl2(PhCN)2 1 Me2N O Me2N O Me2N OH ii. TMSCl reflux 6 4 6 4 71% yield, 1:1 Yamazaki, T.; Ichige, T.; Takei, S.; Kawashita, S.; Kitazume, T.; Kubota, T. Org. Lett. 2001, 3, 2915–2918.
- 28. Confirmation of Chair t-Bu Me t-Bu Si Me O i. AgOTs (10 mol %), HO2C OH HO CO2H Me O Me –25 °C Me Et Me + ii. HF•Pyr, rt OBn O Et OBn Me OBn Me Et HO2C OH H HO2C OH H Me α β Et Me α β H OBn H Me H OBn Me H Et
- 29. Increasing Steric Bulk O O R Me Me O Me O OH O Me HO2C HO2C OP HO2C HO Me HO OP O R Me HO Me
- 30. Increasing Steric Bulk O O R Me Me O Me O OH O Me HO2C HO2C OP HO2C HO Me HO OP O R Me HO Me O Me O Me OBn O t-Bu
- 31. Increasing Steric Bulk t-Bu Me t-Bu Si Me O O i. AgOTs (10 mol %), Me O Me + Me O Me –25 °C OBn O t-Bu OBn O t-Bu ii. HF•Pyr, rt 50 : 50 HO2C OH HO CO2H HO CO2H Me t-Bu Me + Me t-Bu + OBn Me OBn Me t-Bu OBn Me 25 : 25 : 50
- 32. Vicinal Stereocontrol t-Bu t-Bu Si O O HO CO2H Me O O Me O Me Me HO Me O OBn Me O OBn O OBn HO2C Me H Me t-Bu t-Bu Si O O Me HO CO2H Me O O Me O Me HO Me O O O OBn Me OBn O Me Me OBn HO2C HO2C H Me O HO t-Bu t-Bu Si O Me O HO2C OH Me O O Me O Me Me HO2C (+)-latifolic acid Me O O OBn O OBn Me HO BnO Me Me H t-Bu t-Bu O Me Si O HO2C OH Me O O Me O Me HO2C O O OBn O Me OBn Me HO BnO Me Me H
- 33. Lactone Synthesis O HO CO2H conditions HO O R1 R1 R2 R2 X Entry R1 R2 X Conditions Yield d.r. 1 Ph H I NaHCO3, I2, CH3CN, 0 °C 91% 3:2 2 Et n-Bu OH H2O, t-BuOH, AD mix NR - 3 Et n-Bu Br NBS, THF, 0 °C 76% 5:1 4 Et n-Bu I NIS, THF, 0 °C 92% 3:1 5 Et n-Bu OH OsO4, NMO, CH2Cl2 73% 1:0 6 Et n-Bu OH m-CPBA, CH2Cl2 56% 0:1
- 34. Potential Targets HO O HO O Me Me OMe PPh3, I2, Imid, O PMBO O O O O CH2Cl2, reflux HO PMBO H OH HO OH (-) delesserine 95% O Me O Me Me O Me OAc O O Me OH O O Me Me O OH Me Me H Me Me O Me O OAc OH O O Ferupennin F Trilobolide
- 35. Acknowledgements Support Crew UCI Weiss Lab Scott Chiou Dr. Keith Woerpel Dr. Allison Olszewski Mike Ciriza Dr. Gregory Weiss Dr. Aron Levin Lynda Ciriza Dr. David Van Vranken Dr. Sara Chiou LOTC Dr. Birte Feld Dr. Yemi Adesokan Dr. John Greaves Woerpel Lab My eternal roommate: Dr. Matt Dr. Phil Dennison Current and Past Woerpel Morin Dr. Joseph Ziller Members Vicky Cox UCI Staff + Faculty Dr. Tim Clark Chris Carol Laura Bourque Craig Bottolfson Rainier Lab Walter Salamant Tom Campobello Dr. Jon Rainier Joey Georges Dr. Jason Cox Christian Ventocilla Dr. David Colby Dr. Jason Imbriglio Kay Buchner Dr. Rob Bahde Dr. Scott Roberts Dr. Susan Billings Dr. David France Dr. Shawn Allwein Dr. Stacie Calad Dr. Kevin Bahnck Mike Taday Catt Edgley TMS Crew Dr. Ryan Hadley Andrew Thomas DSK Dr. Jennifer Krumper Brandon Maggs Dr. Michael Yang This is a big horse HSN guy Dr. Laura Anderson Rockband 2, Guitar Hero 3 Dr. Pamela Haile Dr. Renee Link Dr. Nick Leonard Dr. Tony Romero
- 36. Acknowledgements Support Crew UCI Weiss Lab Scott Chiou Dr. Keith Woerpel Dr. Allison Olszewski Mike Ciriza Dr. Gregory Weiss Dr. Aron Levin Lynda Ciriza Dr. David Van Vranken Dr. Sara Chiou LOTC Dr. Birte Feld Dr. Yemi Adesokan Dr. John Greaves Woerpel Lab My eternal roommate: Dr. Matt Dr. Phil Dennison Current and Past Woerpel Morin Dr. Joseph Ziller Members Vicky Cox UCI Staff + Faculty Dr. Tim Clark Chris Carol Laura Bourque Craig Bottolfson Rainier Lab Walter Salamant Matt Beaver Tom Campobello Dr. Jon Rainier Joey Georges Dr. Jason Cox Christian Ventocilla Dr. David Colby Dr. Jason Imbriglio Kay Buchner Dr. Rob Bahde Dr. Scott Roberts Dr. Susan Billings Dr. David France Dr. Shawn Allwein Dr. Stacie Calad Dr. Kevin Bahnck Mike Taday Catt Edgley TMS Crew Dr. Ryan Hadley Andrew Thomas DSK Dr. Jennifer Krumper Brandon Maggs Dr. Michael Yang This is a big horse HSN guy Dr. Laura Anderson Rockband 2, Guitar Hero 3 Dr. Pamela Haile Dr. Renee Link Dr. Nick Leonard Dr. Tony Romero
- 37. Acknowledgements Mark and Bonnie Howard Kevin and Kristin Howard Howard, Aunt Julie, Uncle Steve
- 38. CA Peeps
- 39. CA Peeps
- 40. More CA Peeps
- 42. Phx Peeps
- 43. Fam
- 44. Tucson Peeps
Notas del editor
- • sila carbonyl ylide is common intermediate • for rearrangement product, last arrow is not an equilibrium arrow, verify that this is correct. • figure out if you want to use the proposed silyl-silver complex rather than a symbolic one
- -Read these references, how do the generate their silylene? •mechanism from woerpel paper on how they get the d.r. that they do, note the woerpel example forms a new CC bond •basically 1,2 addition across the carbonly to give the oxasilacyclopropane or some derivative of.