Worksheet solubility and solubility rules
•Download as DOC, PDF•
2 likes•1,511 views
Report
Share
Report
Share
Recommended
Recommended
More Related Content
More from Gaby De La Garza
More from Gaby De La Garza (20)
Recently uploaded
Recently uploaded (20)
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
Strategies for Landing an Oracle DBA Job as a Fresher

Strategies for Landing an Oracle DBA Job as a Fresher
Apidays Singapore 2024 - Building Digital Trust in a Digital Economy by Veron...

Apidays Singapore 2024 - Building Digital Trust in a Digital Economy by Veron...
Navi Mumbai Call Girls 🥰 8617370543 Service Offer VIP Hot Model

Navi Mumbai Call Girls 🥰 8617370543 Service Offer VIP Hot Model
Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...

Apidays New York 2024 - Accelerating FinTech Innovation by Vasa Krishnan, Fin...
ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke

ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke
Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe

Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe
"I see eyes in my soup": How Delivery Hero implemented the safety system for ...

"I see eyes in my soup": How Delivery Hero implemented the safety system for ...
AWS Community Day CPH - Three problems of Terraform

AWS Community Day CPH - Three problems of Terraform
Apidays Singapore 2024 - Scalable LLM APIs for AI and Generative AI Applicati...

Apidays Singapore 2024 - Scalable LLM APIs for AI and Generative AI Applicati...
Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...

Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...
Automating Google Workspace (GWS) & more with Apps Script

Automating Google Workspace (GWS) & more with Apps Script
Strategies for Unlocking Knowledge Management in Microsoft 365 in the Copilot...

Strategies for Unlocking Knowledge Management in Microsoft 365 in the Copilot...
Apidays Singapore 2024 - Modernizing Securities Finance by Madhu Subbu

Apidays Singapore 2024 - Modernizing Securities Finance by Madhu Subbu
Worksheet solubility and solubility rules
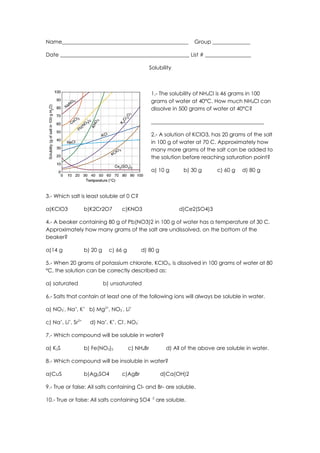
- 1. Name_______________________________________________ Group ______________ Date ________________________________________________ List # _________________ Solubility 1.- The solubility of NH4Cl is 46 grams in 100 grams of water at 40°C. How much NH4Cl can dissolve in 500 grams of water at 40°C? __________________________________________ 2.- A solution of KClO3, has 20 grams of the salt in 100 g of water at 70 C. Approximately how many more grams of the salt can be added to the solution before reaching saturation point? a) 10 g b) 30 g c) 60 g d) 80 g 3.- Which salt is least soluble at 0 C? a)KClO3 b)K2Cr2O7 c)KNO3 d)Ce2(SO4)3 4.- A beaker containing 80 g of Pb(NO3)2 in 100 g of water has a temperature of 30 C. Approximately how many grams of the salt are undissolved, on the bottom of the beaker? a)14 g b) 20 g c) 66 g d) 80 g 5.- When 20 grams of potassium chlorate, KClO3, is dissolved in 100 grams of water at 80 ºC, the solution can be correctly described as: a) saturated b) unsaturated 6.- Salts that contain at least one of the following ions will always be soluble in water. a) NO3-, Na+, K+ b) Mg2+, NO3-, Li+ c) Na+, Li+, Sr2+ d) Na+, K+, Cl-, NO3- 7.- Which compound will be soluble in water? a) K2S b) Fe(NO3)2 c) NH4Br d) All of the above are soluble in water. 8.- Which compound will be insoluble in water? a)CuS b)Ag2SO4 c)AgBr d)Ca(OH)2 9.- True or false: All salts containing Cl- and Br- are soluble. 10.- True or false: All salts containing SO4 -2 are soluble.
