Thermodynamics Hw#2
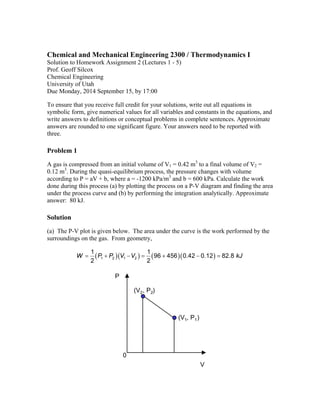
- 1. Chemical and Mechanical Engineering 2300 / Thermodynamics I Solution to Homework Assignment 2 (Lectures 1 - 5) Prof. Geoff Silcox Chemical Engineering University of Utah Due Monday, 2014 September 15, by 17:00 To ensure that you receive full credit for your solutions, write out all equations in symbolic form, give numerical values for all variables and constants in the equations, and write answers to definitions or conceptual problems in complete sentences. Approximate answers are rounded to one significant figure. Your answers need to be reported with three. Problem 1 A gas is compressed from an initial volume of V1 = 0.42 m3 to a final volume of V2 = 0.12 m3. During the quasi-equilibrium process, the pressure changes with volume according to P = aV + b, where a = -1200 kPa/m3 and b = 600 kPa. Calculate the work done during this process (a) by plotting the process on a P-V diagram and finding the area under the process curve and (b) by performing the integration analytically. Approximate answer: 80 kJ. Solution (a) The P-V plot is given below. The area under the curve is the work performed by the surroundings on the gas. From geometry, 1 196 456 0 42 0 12 82 8 2 1 2 1 2 2 W P P V V . . . kJ 0 V P (V2, P2) (V1, P1)
- 2. (b) The work done on the gas can also be calculated analytically as W PdV aV b dV a V V b V V or W . . . . . 2 2 2 2 12 1 1 1 2 1 2 2 2 12 2 1200 0 42 0 12 600 0 42 0 12 82 8 kJ 2 Problem 2 A diaphragm divides a rigid, well insulated, 2-m3 tank into two equal parts. The left side contains nitrogen gas (N2) at 10 bar (absolute) and 300 K. The right side contains nothing, it is a vacuum. A small hole forms in the diaphragm and gas slowly leaks out from the left side. After some time the temperature in the whole tank is uniform. What is the final temperature? You may assume ideal gas behavior. Approximate answer: 300 K. Solution The closed system is sketched here and includes both chambers. Assume ideal gas behavior and neglect changes in kinetic and potential energy. There is no heat transfer or work. 1 m3 N2 10 bar 300 K 1 m3 initially empty The integrated energy balance for our closed system is 2 1 , , ( ) 0 in net in net U m u u W Q The internal energy of an ideal diatomic is 5 2 u RT and it depends only on temperature. Because the internal energy of the system is constant, T2 = T1 = 300 K. Problem 3 Fifty grams of air are compressed isothermally at 300 K from 1 bar to 10 bar (absolute pressures). Calculate the work done on the air (kJ). Approximate answer: 10 kJ.
- 3. Solution Apply the ideal gas model, PV = mRT, and calculate the boundary work done on the system as follows. W 2 PdV mRT lP 1 P n2 1 8.3145 kPa m3 0.050 kg* *300 K*ln(10) = 9.90 kJ 29 kg K V in V Problem 4 Fifty grams of oxygen in a piston-cylinder assembly are compressed – not adiabatically, not isothermally, but somewhere in between per the relationship PV1.2 constant from 100 kPa (absolute) and 300 K to 1 MPa (absolute). Find the heat transferred (kJ), if any, to or from the system during this process. If there is heat transfer, is it positive or negative? You may assume ideal gas behavior. Approximate answer: Qin = - 5 kJ. Solution
- 4. Problem 5 The inner and outer surfaces of a 5-m-by-6-m brick wall of thickness 30 cm and thermal conductivity 0.69 W/(m C) are steady temperatures of 20C and 5C. Determine the rate of heat transfer through the wall, in W. Note that 0.69 W/(m C) = 0.69 W/(m K). Approximate answer: 1000 W. Solution From (2-51),
- 5. 0.69 W 30 m2 20 5°C 1040 W m°C 0.30 m Q kA T x Problem 6 Objects radiate and absorb radiant energy. Suppose we have an unclothed person with surface area A = 1.8 m2, a skin temperature of T1 = 34ºC, in a room with surfaces at T2 = 15ºC. Approximating the person as a blackbody radiator, find the net rate of heat loss by radiation (watts) from the equation 4 4 net 1 2 Q A T T where the Stefan-Boltzmann constant, = 5.67x10-8 W/(m2 K4). Approximate answer: 200 W. Solution The net rate of heat loss by radiation is 4 4 1 2 ( ) net Q A T T Because the skin is nearly black, = 1 and Q 5.67x10 W 1.8m 34 273.15 (15 273.15 ) K 205W 8 2 4 4 4 net 2 4 m K Note that the temperatures must be absolute (kelvin scale for this problem). The rate of heat loss by radiation is about twice the resting metabolic rate of an adult. Additional heat loss will occur by natural convection. Our person needs to get dressed or start exercising to avoid feeling cold. Problem 7 An electric water heater holds water at 135ºF in a room at 65ºF. Its insulation is equivalent to R5 (R-value = 5). The owner fits a 25-ft2, R10 blanket on the water heat, raising its total R value to 15. If the conversion of electricity into heated water is 100%, how much energy (kWhr) will be saved each year? If electricity costs 7.6 cents/kWhr, how much money will be saved each year? Approximate answers: 600 kWh/yr and $50/yr. Solution The rate of heat loss is
- 6. i o A T T Q R The energy saved during the interval t = 1 yr is Q Q t A T T 1 1 t old new i o R R old new 25 ft 135 65 °F 1 1 Btu 365 day 24 h 1kWh 599.1 kWh 2 2 5 15 °F ft h yr day 3412Btu yr The money saved in one year is 599.1 kWh $0.076 $45.53 / yr yr kWh