Periodic table
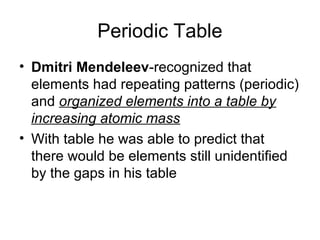
- 1. Periodic Table • Dmitri Mendeleev-recognized that elements had repeating patterns (periodic) and organized elements into a table by increasing atomic mass • With table he was able to predict that there would be elements still unidentified by the gaps in his table
- 2. • Henry Moseley - determined that the number of protons - atomic number (which is unique to each element) would allow the elements to fit into very specific pattern • All identified elements follow the periodic law – chemical and physical properties change periodically with atomic number
- 3. Metals • Most elements are metals • Found to the left of the zigzag line • Solid at room temp (exception: mercury and hydrogen – nonmetal) • Properties: – Shiny – Ductile – Malleable – Good conductors
- 4. Metalloids • Also called semiconductors • Border the zigzag line (exception Al) • Have properties of both metals and nonmetals depending on the conditions • properties: depending on conditions – Brittle – Good conductors – Some shiny (others dull)
- 5. nonmetals • More than half are gases at room temp • To the right of the zigzag line • Properties: • Not malleable or ductile • Not shiny or dull • Poor conductors
- 6. Each square on table • Each square includes: • elements name • chemical symbol (color coded to identify if element is a solid, liquid or gas at room temp) • Atomic number (protons) • Atomic mass • Background color (identifies metals, nonmetals and metalloids on table)
- 7. • First letter of chemical symbol is always upper case and any additional letters are lower case • Newest elements have temporary 3 letter symbols • Rows (left to right) are called periods- determines the number of energy levels • Properties gradually change moving left to right across each row from reactive (group 1) to non- reactive (group 18)
- 8. • Columns are called groups or family • Elements in the same group or family have similar properties moving up and down each column • Each element in a family has the same number of valence electrons in the outer shell • Group number determine the valence electrons (ex: group one – all elements in group 1 have 1 valence electron)
- 9. Energy Levels • 1st energy level – 2 valence electrons (max) • 2nd energy level – 8 valence electrons (max) • 3rd energy level – 8 valence electrons (max) • And so on…. • Each energy level can have less valence electrons but they can not have more than the maximum valence electrons.
- 10. Bonds • To form bonds, elements must reach a full state of 8 valence electrons in the outermost energy level (octet rule) (Exception: would be first energy level which is full at 2-helium)
- 11. Group 1: Alkali metals • Metals • 1 valence electron in outer level (easily shared and form compounds easily) • Very reactive with H2O, O2 and other elements • Don’t appear in nature by themselves, only as compounds
- 12. Group 2 – Alkaline-Earth Metals • Metals • 2 valence electrons in outer level (slightly less reactive)
- 13. Group 3 – 12: Transition • Metals • 1 or 2 valence electrons in outer level (depending on element) and are less reactive
- 14. Lanthanides and Actinides • In periods 6 and 7 and appear at the bottom of the periodic table to keep table from being to wide • Lanthanides are shiny reactive metals • Actinides are unstable radioactive • All elements after Pu-94 (plutonium) are man-made in labs and don’t occur in nature
- 15. Group 13: Boron Group • Has 1 metalloid and 4 metals • 3 valence electrons in outer level and are semi reactive
- 16. Group 14-Carbon group • 1 nonmetal, 2 metalloids and 2 metal • 4 valence electrons in outer level and most non-reactive depending on element • Forms organic compounds (all living things contain carbon)
- 17. Group 15-Nitrogen Group • 2 nonmetals, 2 metalloids, 1 metal • 5 valence electrons in outer level and reactivity depends on conditions and element • P is extremely reactive and only appears in compounds
- 18. Group 16-Oxygen Group • 3 nonmetals, 1 metalloid, and 1 metal • 6 valence electrons in outer level and reactivity depends on element – Po-84 is radioactive
- 19. Group 17-Halogens • Nonmetals • 7 valence electrons in outer level and has violent reactions with alkali-metals to form salt compounds – Highly reactive with other elements – Do not appear in nature alone only in compounds
- 20. Group 18-Noble Gases • Nonmetals • 8 valence electrons in outer level (full level) (except helium which has 2 valence electrons, which makes helium full) and very un-reactive – inert • Do not form compounds under normal conditions
- 21. Hydrogen • Nonmetal • 1 electron in outer level so it is set above the alkali metals and is reactive • Properties: even though above metal category, has properties of nonmetals • Most abundant element in universe, makes up stars
- 22. • Atomic number = Number of Protons • Electrons equal to the number of protons • Neutrons equal atomic mass minus the protons • Protons do not change in a atom, neutrons can change, electrons can be shared or transferred (when bonds are made)