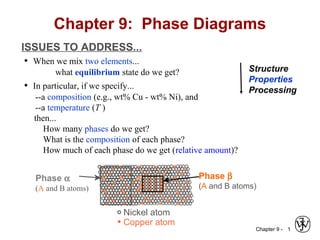
Ch09 m
- 1. Chapter 9 - 1 ISSUES TO ADDRESS... • When we mix two elements... what equilibrium state do we get? • In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) then... How many phases do we get? What is the composition of each phase? How much of each phase do we get (relative amount)? Chapter 9: Phase Diagrams Phase β (A and B atoms) Phase α (A and B atoms) Nickel atom Copper atom Structure Properties Processing
- 2. Chapter 9 - 2 Phase Equilibria: Review: Solubility Limit Introduction - Solutions – solid solutions, single phase (e.g. syrup) - Mixtures – more than one phase (syrup and solid sugar) Phase: Homogeneous portion of a system that has uniform physical and chemical properties Solubility Limit: Max concentration for which only a single phase solution occurs. Q: What is the solubility limit at 20°C? Answer: 65 wt% sugar. If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure Sugar Temperature(°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid sugar) 20 40 60 80 100 Pure Water
- 3. Chapter 9 - 3 • Components: The elements or compounds which are present in the mixture (e.g., Al and Cu) • Phases: The physically and chemically distinct material regions that result (e.g., α and β). •Equilibrium No change with time – fixed composition for each component Al-Cu Alloy Components and Phases α (darker phase) Includes Al and Cu β (lighter phase) Includes Al and Cu Two phases Two Components
- 4. Chapter 9 - 4 Effect of T & Composition (Co) • Changing T can change # of phases: D (100°C,90) 2 phases B (100°C,70) 1 phase path A to B. • Changing Co can change # of phases: path B to D. A (20°C,70) 2 phases 70 80 1006040200 Temperature(°C) Co =Composition (wt% sugar) L (liquid solution i.e., syrup) 20 100 40 60 80 0 L (liquid) + S (solid sugar) water- sugar system
- 5. Chapter 9 - 5 Phase Diagrams Types of phase diagrams depend on Solubility • Isomorphous phase diagram (complete solubility) • Eutectic phase diagram (partial solubility) • Complex phase diagram (…….) How to obtain phase diagrams ? - From cooling curves for various starting solutions (Thermal method) • Thermodynamics: Phase Diagrams Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). Phase Diagram: A Plot of T versus C indicating various boundaries between phases T Time ? Phase Change T composition Uses of Phase Diagrams: - No. of phases - Composition of each phase - Relative amount of each phase + Predict the microstructure
- 6. Chapter 9 - 6 Phase Equilibria - Solutions Crystal Structure electroneg r (nm) Ni FCC 1.9 0.1246 Cu FCC 1.8 0.1278 Both metals have •Nearly the same atomic radius •The same crystal structure (FCC) •similar electronegativities (W. Hume – Rothery rules) suggesting high mutual solubility. Simple Solution System (e.g., Ni-Cu solution) Remember Ni and Cu are totally miscible (soluble) in all proportions (compositions). ONE PHASE ALWAYS – Isomorphous !
- 7. Chapter 9 - 7 Phase Diagrams- Metals • 2 phases: L (liquid) α (FCC solid solution) • 3 phase fields: L L + α α Phase Diagram for Cu-Ni system 20 40 60 80 1000 wt% Ni 1000 1100 1200 1300 1400 1500 1600 T(°C) L (liquid) α (FCC solid Soln) L +αLiquidus line Solidus line Isomorphous Phase Diagram Complete solubility - one phase field extends from 0 to 100 wt% Ni.
- 8. Chapter 9 - 8 Phase Change • Phase boundary: Lines between different phases e.g. – Liquid – solid • one phase • two phases …etc) • Phase Change: – Solidification (or melting) – Phase Reaction: one phase gives DIRECTLY two phases e.g. Liquid Solid phase 1 + Solid Phase 2 (both insoluble) • This occurs at – Certain (fixed) temperature: TE – Certain (fixed) composition: CE • Eutectic Phase Reaction: L α + β • There are other types of phase reactions (Later)
- 9. Chapter 9 - 9 Cooling Curves There are various types of cooling curves: Depending on composition (characteristics) 1. Pure Element 2. Mixture of soluble 2 elements 3. Mixture of Partially Soluble 2 elements 4. Mixtures undergoing Phase Reactions 1. At the composition for phase reaction 2. Before the composition for phase reaction
- 10. Chapter 9 - 10 Types of Cooling Curves 2) Mixture of soluble 2 elements Time L L α α T 3) Phase Reaction at CE 3) Phase Reaction: Co > CE Time L L α + β α + β T L α T Time L L α (A or B) α 1) Pure Element Tmelting Time L L α + β α + β T TE
- 11. Chapter 9 - 11 Constructing Phase Diagrams Thermal Method • Start with several different solutions • Each solution at certain co • Prepare the solutions in liquid form (melting) • Cool each solution while recording T versus time • Plot the cooling curve for each solution • At each phase change: there are changes in the slope – Latent Heat – Change in the properties (specific heat) – Phase reaction (later) • Project cooling curves on T versus co plot. • Connect the points from projection creating boundaries. • Label different obtained areas and boundaries.
- 12. Chapter 9 - 12 Constructing Isomorphous Phase Diagrams Cooling Curves at Various Initial Co Phase Diagram T verus Co T Composition Time
- 13. Chapter 9 - 13 wt% Ni20 40 60 80 1000 1000 1100 1200 1300 1400 1500 1600 T(°C) L (liquid) α (FCC solid solution) L + α liquidus solidus Cu-Ni phase diagram Using Phase Diagrams: # and types of phases Rule 1: If we know T and Co, then we know: - Number and types of phases present. • Examples: A(1100°C, 60): 1 phase: α B(1250°C, 35): 2 phases: L + α B(1250°C,35) A(1100°C,60)
- 14. Chapter 9 - 14 wt% Ni 20 1200 1300 T(°C) L (liquid) α (solid)L +α liquidus solidus 30 40 50 L +α Cu-Ni system Using Phase Diagrams: composition of phases Rule 2: If we know T and Co, then we know: - the composition of each phase. • Examples: TA A 35 Co 32 CL At TA = 1320°C: Only Liquid (L) CL = Co ( = 35 wt% Ni) At TB = 1250°C: Both α and L CL = Cliquidus ( = 32 wt% Ni here) Cα = Csolidus ( = 43 wt% Ni here) At TD = 1190°C: Only Solid ( α) Cα = Co ( = 35 wt% Ni) Co = 35 wt% Ni B TB D TD tie line 4 Cα 3
- 15. Chapter 9 - 15 Rule 3: If we know T and Co, then we know: --the relative amount of each phase (given in wt%). • Examples: At TA: Only Liquid (L) WL = 1.0 , W =α = 0 At TD: Only Solid ( α) WL = 0, Wα = 1.0 Co = 35 wt% Ni Using Phase Diagrams: weight fractions of phases wt% Ni 20 1200 1300 T(°C) L (liquid) α (solid)L +α liquidus solidus 30 40 50 L +α Cu-Ni system TA A 35 Co 32 CL B TB D TD tie line 4 Cα 3 R S At TB: Both α and L 73.0 3243 3543 = − − = = 0.27 WL = S R +S Wα = R R +S
- 16. Chapter 9 - 16 • Tie line – connects the phases in equilibrium with each other - essentially an isotherm The Lever Rule How much of each phase? ThinkThink of it as a lever (teeter-totter) ML Mα R S RMSM L ⋅=⋅α L L LL L L CC CC SR R W CC CC SR S MM M W − − = + = − − = + = + = α α α α α 00 wt% Ni 20 1200 1300 T(°C) L (liquid) α (solid)L +α liquidus solidus 30 40 50 L +α B TB tie line CoCL Cα SR
- 17. Chapter 9 - 17 wt% Ni 20 1200 1300 30 40 50 1100 L (liquid) α (solid) L + α L + α T(°C) A 35 Co L: 35wt%Ni Cu-Ni system Observe the microstructure while going down (cooling) at the same composition. • Consider Co = 35 wt%Ni. Microstructure - Cooling in a Cu-Ni Binary 4635 43 32 α: 43 wt% Ni L: 32 wt% Ni L: 24 wt% Ni α: 36 wt% Ni Bα: 46 wt% Ni L: 35 wt% Ni C D E 24 36
- 18. Chapter 9 - 18 • Cα changes as we solidify. • Cu-Ni case: • Fast rate of cooling: Cored structure • Slow rate of cooling: Equilibrium structure First α to solidify has Cα = 46 wt% Ni. Last α to solidify has Cα = 35 wt% Ni. Cored vs Equilibrium Phases First α to solidify: 46 wt% Ni Uniform Cα: 35 wt% Ni Last α to solidify: < 35 wt% Ni
- 19. Chapter 9 - 19 Mechanical Properties: Cu-Ni System • Effect of solid solution strengthening on: --Tensile strength (TS) - Peak as a function of Co TensileStrength(MPa) Composition, wt% Ni Cu Ni 0 20 40 60 80 100 200 300 400 TS for pure Ni TS for pure Cu --Ductility (%EL,%AR) - Min. as a function of Co Elongation(%EL) Composition, wt% Ni Cu Ni 0 20 40 60 80 100 20 30 40 50 60 %EL for pure Ni %EL for pure Cu
- 20. Chapter 9 - 20 Eutectic Phase Diagram With Eutectic Phase Reaction L(CE) α(CαE) + β(CβE) The phase diagram is characterized with: • 3 single phase regions (L, α and β) • Partial Solubility – Solubility Limit of A in B forming β B in A forming α Occurs at L (liquid) α L + α L+ββ α + β Wt% A: Co 1000 T CE TE : Eutectic Composition• CE • TE : Eutectic Temperature Remember Phase Reaction at CE Time L L α + β α + β T TE
- 21. Chapter 9 - 21 Eutectic Phase Diagram – Cooling Curves L α L+α L+ββ α+β Wt% A: Co 1000 T(°C) T Time L L α α 1) Pure A or B Tmelting 2) Giving α or β Time L L α α T Time L L α + β α + β T L α Time L L α + β α + β T TE What is the difference in this side
- 22. Chapter 9 - 22 Maximum solubility of A in B L α L + α L+ββ α + β Wt% A: Co 1000 T(°C) Liquidus Solidus Solvus Eutectic Reaction Eutectic Phase Diagram Maximum solubility of B in A Boundaries - lines: •Liquidus •Solidus •Solvus Now, apply previous rules ? On real systems
- 23. Chapter 9 - 23
- 24. Chapter 9 - 24
- 25. Chapter 9 - 25 Example-Eutectic System α: mostly Cu β: mostly Ag • TE : No liquid below TE Ex.: Cu-Ag system L (liquid) α L + α L+ββ α + β Co , wt% Ag 20 40 60 80 1000 200 1200 T(°C) 400 600 800 1000 CE TE 8.0% CE=71.9% 91.2% TE=779°C Given T and C Values are characteristics Of Cu-Ag system
- 26. Chapter 9 - 26 L+α L+β α + β 200 T(°C) 18.3 C, wt% Sn 20 60 80 1000 300 100 L (liquid) α 183°C 61.9 97.8 β • For a 40 wt% Sn-60 wt% Pb alloy at 150°C, find... --the phases present: Pb-Sn system EX: Pb-Sn Eutectic System (1) α + β --compositionscompositions of phases: CO = 40 wt% Sn --the relative amountrelative amount of each phase: 150 40 Co 11 Cα 99 Cβ SR Cα = 11 wt% Sn Cβ = 99 wt% Sn Wα= Cβ - CO Cβ - Cα = 99 - 40 99 - 11 = 59 88 = 0.67 S R+S = Wβ = CO - Cα Cβ - Cα = R R+S = 29 88 = 0.33 (or 1 – 0.67)= 40 - 11 99 - 11
- 27. Chapter 9 - 27 L+β α + β 200 T(°C) C, wt% Sn 20 60 80 1000 300 100 L (liquid) α β L+α 183°C • For a 40 wt% Sn-60 wt% Pb alloy at 200200°°CC, find... --the phases present: Pb-Sn system EX: Pb-Sn Eutectic System (2) α + L --compositionscompositions of phases: CO = 40 wt% Sn --the relative amountrelative amount of each phase: Wα = CL - CO CL - Cα = 46 - 40 46 - 17 = 6 29 = 0.21 WL = CO - Cα CL - Cα = 23 29 = 0.79 40 Co 46 CL 17 Cα 220 SR Cα = 17 wt% Sn CL = 46 wt% Sn
- 28. Chapter 9 - 28 • Co < 2 wt% Sn • Result: --at extreme ends --polycrystal of α grains i.e., only one solid phase. Microstructures in Eutectic Systems: I 0 L+ α 200 T(°C) Co, wt% Sn 10 2 20 Co 300 100 L α 30 α+β 400 (room T solubility limit) TE (Pb-Sn System) α L L: Co wt% Sn α: Co wt% Sn Depends on the composition
- 29. Chapter 9 - 29 • 2 wt% Sn < Co < 18.3 wt% Sn • Result: Initially liquid + α then α alone finally two phases α polycrystal fine β-phase inclusions Microstructures in Eutectic Systems: II Pb-Sn system L + α 200 T(°C) Co , wt% Sn 10 18.3 200 Co 300 100 L α 30 α+ β 400 (sol. limit at TE) TE 2 (sol. limit at Troom) L α L: Co wt% Sn α β α: Co wt% Sn
- 30. Chapter 9 - 30 • Co = CE • Result: Eutectic microstructure (lamellar structure) --alternating layers (lamellae) of α and β crystals. Microstructures in Eutectic Systems: III 160µm Micrograph of Pb-Sn eutectic microstructure Pb-Sn system L+β α + β 200 T(°C) C, wt% Sn 20 60 80 1000 300 100 L α β L+α 183°C 40 TE 18.3 α: 18.3 wt%Sn 97.8 β: 97.8 wt% Sn CE 61.9 L: Co wt% Sn
- 31. Chapter 9 - 31 Lamellar Eutectic Structure
- 32. Chapter 9 - 32 • 18.3 wt% Sn < Co < 61.9 wt% Sn • Result: α crystals and a eutectic microstructure Microstructures in Eutectic Systems: IV 18.3 61.9 SR 97.8 SR primary α eutectic α eutectic β Pb-Sn system L+β200 T(°C) Co, wt% Sn 20 60 80 1000 300 100 L α β L+α 40 α+β TE L: Co wt% Sn Lα L α
- 33. Chapter 9 - 33 • 18.3 wt% Sn < Co < 61.9 wt% Sn • Result: α crystals and a eutectic microstructure Microstructures in Eutectic Systems: IV 18.3 61.9 SR 97.8 SR primary α eutectic α eutectic β WL = (1-Wα) = 0.50 Cα = 18.3 wt% Sn CL = 61.9 wt% Sn S R + S Wα‘ = = 0.50 • Just above TE : • Just below TE : Cα = 18.3 wt% Sn Cβ = 97.8 wt% Sn S R + S Wα= = 0.73 Wβ = 0.27 Pb-Sn system L+β200 T(°C) Co, wt% Sn 20 60 80 1000 300 100 L α β L+α 40 α+β TE L: Co wt% Sn Lα L α What is the fraction of Eutectic α ? Pro-eutectic (primary) α ?
- 34. Chapter 9 - 34 L+α L+β α + β 200 Co, wt% Sn20 60 80 1000 300 100 L α β TE 40 (Pb-Sn System) Hypoeutectic & Hypereutectic 160 µm eutectic micro-constituent hypereutectic: (illustration only) β β β β β β 175 µm α α α α α α hypoeutectic: Co = 50 wt% Sn T(°C) 61.9 eutectic eutectic: Co =61.9wt% Sn
- 35. Chapter 9 - 35 Intermetallic Compounds Note: intermetallic compound forms a line - not an area like α ? Mg2Pb In Previous Phase diagrams α and β are terminal solid solutions Can we obtain other intermediate phases ? Now: Mg2Pb • is an intermetallic compound • with a ratio (2:1) (Mg:Pb)-Molar • melts at a fixed temperature M • Cooling curve ??? Because stoichiometry (i.e. composition) is exact: Calculate ? Calculate Wt% Pb = 207 /(207+2(24.3)) X 100 = 81 %
- 36. Chapter 9 - 36 Same Story ? • No. and type of each phase • Composition of each phase • Relative amount or fraction of each phase • Distinguish between – primary phase – Eutectic phase • Microstructure • Cooling Curves • Fill the regions with types of phases?
- 37. Chapter 9 - 37 Eutectoid & Peritectic Phase Reactions • Eutectic - liquid in equilibrium with two solids L α + βcool heat intermetallic compound - cementite cool heat • Eutectoid - solid phase in equilibrium with two solid phases S2 S1+S3 γ α + Fe3C (727ºC) cool heat • Peritectic - liquid + solid 1 solid 2 (Fig 9.21) S1 + L S2 δ + L γ (1493ºC)
- 38. Chapter 9 - 38 Complex Phase Diagrams α and η are terminal solid solutions - β, β’, γ, δ and ε are intermediate solid solutions. • new phenomena exist: ……… reaction ………. reaction
- 39. Chapter 9 - 39 Eutectoid & Peritectic Cu-Zn Phase diagram Eutectoid transition δ γ + ε Peritectic transition γ + L δ
- 40. Chapter 9 - 40 Phase Diagram with Eutectoid Phase Reactions The eutectoid (eutectic-like in Greek) reaction is similar to the eutectic This phase diagram includes both: Eutectic ReactionEutectoid Reaction Our Interest In Fe-C phase diagram
- 41. Chapter 9 - 41 Iron-Carbon (Fe-C) Phase Diagram • 2 important points -Eutectoid (B): γ ⇒ α +Fe3C -Eutectic (A): L ⇒ γ +Fe3C Fe3C(cementite) 1600 1400 1200 1000 800 600 400 0 1 2 3 4 5 6 6.7 L γ (austeniteaustenite) γ+L γ+Fe3C α+Fe3C α+γ L+Fe3C δ (Fe) Co, wt% C 1148°C T(°C) α 727°C = Teutectoid A SR 4.30 Result: Pearlite = alternating layers of α and Fe3C phases 120 µm γ γ γγ R S 0.76 CeutectoidB Fe3C (cementite-hardhard) α(ferriteferrite-softsoft)
- 42. Chapter 9 - 42 Development of Microstructure in Iron-Carbon alloys PearlitePearlite Structure By DiffusionDiffusion Note: remember the names! Austenite, ferriteferrite, cemetitecemetite and pearlitepearlite
- 43. Chapter 9 - 43 Hypoeutectoid Steel Fe3C(cementite) 1600 1400 1200 1000 800 600 400 0 1 2 3 4 5 6 6.7 L γ (austenite) γ+L γ + Fe3C α+ Fe3C L+Fe3C δ (Fe) Co, wt% C 1148°C T(°C) α 727°C (Fe-C System) C0 0.76 proeutectoid ferritepearlite 100 µm HypoeutectoidHypoeutectoid steel R S α wα =S/(R+S) wFe3C =(1-wα) wpearlite = wγ pearlite r s wα =s/(r+s) wγ =(1- wα) γ γ γ γα α α γγ γ γ γ γ γγ
- 44. Chapter 9 - 44 Hypereutectoid Steel Fe3C(cementite) 1600 1400 1200 1000 800 600 400 0 1 2 3 4 5 6 6.7 L γ (austenite) γ+L γ +Fe3C α +Fe3C L+Fe3C δ (Fe) Co, wt%C 1148°C T(°C) α (Fe-C System) 0.76 Co proeutectoid FeFe33CC 60 µmHypereutectoid steel pearlitepearlite R S wα =S/(R+S) wFe3C =(1-wα) wpearlite = wγ pearlite sr wFe3C =r/(r+s) wγ =(1-w Fe3C ) Fe3C γγ γ γ γγ γ γ γγ γ γ
- 45. Chapter 9 - 45 Phases in Fe–Fe3C Phase Diagram α -ferrite - solid solution of C in …….. Fe • Stable form of iron at room temperature. • The maximum solubility of C is 0.022 wt% (interstitial solubility) • Soft and relatively easy to deform + Fe-C liquid solution γ -austenite - solid solution of C in …….. Fe The maximum solubility of C is 2.14 wt % at 1147°C. Interstitial lattice positions are much larger than ferrite (higher C%) Is not stable below the eutectic temperature (727 °C) unless cooled rapidly (Chapter 10). δ -ferrite solid solution of C in ……. Fe The same structure as α -ferrite Stable only at high T, above 1394 °C Also has low solubility for carbon (BCC) Fe3C (iron carbide or cementite) This intermetallic compound is metastable, it remains as a compound indefinitely at room T, but decomposes (very slowly, within several years) into α -Fe and C (graphite) at 650 - 700 °C
- 46. Chapter 9 - 46 Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following a) composition of Fe3C and ferrite (α) b) the amount of carbide (cementite) in grams that forms per 100 g of steel c) the amount of pearlite and proeutectoid ferrite (α)
- 47. Chapter 9 - 47 Example – Fe-C System g3.94 g5.7CFe g7.5100 022.07.6 022.04.0 100x CFe CFe 3 CFe3 3 3 =α = = − − = − − = α+ α α x CC CCo b) the amount of cementite in grams that forms per 100 g of steel a) composition of Fe3C and ferrite (α) CO = 0.40 wt% C Cα = 0.022 wt% C CFe C = 6.70 wt% C 3 FeC(cementite) 1600 1400 1200 1000 800 600 400 0 1 2 3 4 5 6 6.7 L γ (austenite) γ+L γ + Fe3C α + Fe3C L+Fe3C δ Co, wt% C 1148°C T(°C) 727°C CO R S CFe C3Cα
- 48. Chapter 9 - 48 Chapter 9 – Phase Equilibria c. the amount of pearlite and proeutectoid ferrite (α) note: amount of pearlitepearlite = amount of γγ just abovejust above TE Co = 0.40 wt% C Cα = 0.022 wt% C Cpearlite = Cγ = 0.76 wt% C γ γ+α = Co −Cα Cγ−Cα x 100 =51.2 g pearlite = 51.2 g proeutectoid α = 48.8 g FeC(cementite) 1600 1400 1200 1000 800 600 400 0 1 2 3 4 5 6 6.7 L γ (austenite) γ+L γ + Fe3C α + Fe3C L+Fe3C δ Co, wt% C 1148°C T(°C) 727°C CO R S CγCα Pearlite include ? Eutectoid Fe3CFe3C + Eutectoid ferriteferrite αα
- 49. Chapter 9 - 49 Alloying Steel with More Elements • Teutectoid changes: • Ceutectoid changes: TEutectoid(°C) wt. % of alloying elements Ti Ni Mo Si W Cr Mn wt. % of alloying elements Ceutectoid(wt%C) Ni Ti Cr Si Mn W Mo
- 50. Chapter 9 - 50 • Phase diagrams are useful tools to determine: --the number and types of phases, --the wt% of each phase, --and the composition of each phase for a given T and composition of the system. • Alloying to produce a solid solution usually --increases the tensile strength (TS) --decreases the ductility. • Binary eutectics and binary eutectoids allow for a range of microstructures. Summary