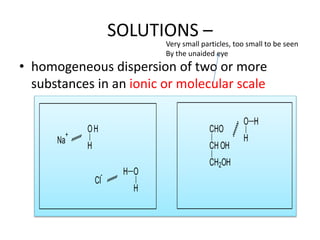
Solutions –
- 1. SOLUTIONS – • homogeneous dispersion of two or more substances in an ionic or molecular scale Na + OH H Cl - H O H CHO CH OH CH2OH O H H Very small particles, too small to be seen By the unaided eye
- 2. Kinds of Mixture Particle Size suspension colloid solution 10-4 cm. in diameter 10-5 – 10-6 cm. in diameter 10-7 cm. in diameter Always suspended in the medium (too small to be pulled down by gravity) Shows Tyndall Effect : reflect light to produce a visible beam of light Always suspended in the medium ( too small to be pulled down by gravity) Settles upon standing
- 3. Kinds of Solutions Solid state Alloys ( Coin, brass) Liquid state Sea water, alcohol in water Dissolved Oxygen (DO) Gaseous state Air
- 4. Parts of a Solution solution solute solvent Dissolved substance Dissolving medium is is
- 5. Parts of a solution Physical state amount Solute Dissolved substance Solid, gas Less Solvent Dissolving medium liquid more
- 6. Nature of Dissolving Process: Na + Na + Na + Na + Cl - Cl - Cl - Cl - H O H Cl - H2O H2O H2O H2O solvated iondissolving rate crystallizing rate salt
- 7. Nature of the Dissolving Process Na+1 Na+1 Na+1 Cl-1 Na+1 Cl-1 Cl-1Cl-1 Cl-1 Na+1 H2O H2O H2O H2O Dissolving rate Crystallizing rate salt Solvated ion
- 8. Nature of Dissolving Process: • The nature of the dissolving process is • a 2 way process 1. Process of dissolution 2. Process of crystallization
- 9. Which, at one point in time the 2 processes will attain equilibrium • At equilibrium • Rate of dissolution == rate of crystallization • Solution is called saturated solution • Concentration of the solution is constant • corresponds to the solubility of the solute in question . •
- 10. Solubility – • maximum amount of solute that will be dissolved by a given amount of solvent producing a stable system, under a specified temperature
- 11. Factors affecting Solubility Nature of solute and solvent : • Like Dissolves Like IMFA solute &solvent IMFA solute IMFA solvent
- 12. Temperature Heat of solution Solid in liquid Gas in liquid T increases solubility increases Endothermic T increases solubility decreases exothermic exothermic T increases solubility decreases
- 13. Pressure • Pressure –has little or no effect on the solubility of solid in liquid, liquid in liquid • Gas in liquid : Pressure increases, solubility increases
- 14. When can a gas become soluble in water ? gas liquid For the gas to become soluble in the liquid , it must come into contact with the liquid: IMFA forming ( exothermic heat flow ) Applied Pressure: Pressure increases, solubility of gas in water increases And this will be effectedby applying pressure to the gas so that IMFA is formed between the gas and water. A process which involves IMFA forming results for heat To flow out to the sorrounding.
- 15. Factors affecting Rate of Dissolution 1. Temperature : As temperature increases, rate of dissolving increases. 2. Stirring increases rate of dissolving 3. Surface area – As surface area increases, rate of dissolving increases
- 16. Concentration = is the amount of solute present in a given amount of solvent producing a solution Described qualitatively quantitatively dilute concentrated saturated unsaturated supersaturated % Molarity (M) Normality (N) Molality (m) Mole fraction (X)
- 17. 1. Dilute- contains a relatively low amount of solute 2. Concentrated – contains a relatively high amount of solute 3. Saturated – contains the maximum amount of solute that can be dissolved by a measured amount of solvent (solubility equivalent ) 4. Unsaturated – one which contains solute concentration lower than the concentration in the saturated solution. 5. Supersaturated – one which contain solute concentration higher than the concentration in the saturated Solution.
- 19. 46.5 g NaAc/100 g H2O 25 C solubility 80 g NaAC/ 100 g H20 50 C all 80 g solute dissolves cool to 25 C all 80 g NaAc is in water as a solution indefinitely called supersaturated solution which can be destroyed by seeding agitation Saturated supersaturated If amount of NaAc is < 46.5 g in 100 g H2O At 25 oC Unsaturated solution Saturated solution With undissolved solute
- 20. A B C At the start of Dissolving Solute amount in Solvent is zero During dissolving Solute amount in solvent Has increased, but still less Than the dissolving rate After dissolving Solute amount in solvent Has increased so that dissolving Rate is equal to the crystallizing rate At what point during the dissolving rate is the saturated solution ? C Not a solution Unsaturated solution Saturated solution
- 21. QUANTITATIVE METHODS OF EXPRESSING CONCENTRATION OF SOLUTION SOLUTION SOLUTE SOLVENT/WATER % Molarity Normality molality Mole fraction
- 22. PerCent = Part Quantity/Total Quantity X 100 1. Percent by mass = grams ofsolute/grams of solution X 100 2. Percent by volume = volume of solute/volume of solution X 100 3. Percent by mass-volume = grams of solute/volume of solution X 100
- 23. A 0.50 liter bottle of wine contains 60 ml ethanol. What is the % v/v ethanol in the solution ? SOLUTION SOLUTE SOLVENT 0.50 liter 60 ml volume solute % = ------------------- X 100 volume solution
- 24. How many grams of KCl are required to prepare 250 grams of an aqueous solution that is 10.0 % KCl by mass SOLUTION SOLUTE SOLVENT ? 250 grams means 10 g KCl = 100 g solution Conversion factor
- 26. What is the M of a 10 % HCl solution of a density of 1.2 g /ml SOLUTION SOLUTE SOLVENT 10 g HCl = 100 g solution 10g HCl = 100 g soluton means M = wt/mwt/liter
- 27. NORMALITY (N) SOLUTION SOLUTE SOLVENT/WATER Number of equivalents ( (wt/MW ) X F ) Number of milliequivalents ( (wt/MW) X F X 1000) LITER milliliter
- 28. Mole fraction (X) SOLUTION SOLUTE SOLVENT/WATER mole MoleAdd to Mole total Mole solute/mole total
- 30. Calculate the molality of a solution of 2.34 g acetic acid, HC2H3O2 in 35.0 g water SOLUTION SOLUTE SOLVENT 2.34 g HC2H3O2 35.0 g MWT = 60.0 g/mole wt/ MWT solute m = ------------------- Kg solvent
- 31. DILUTION • = process of adding water to a solution of known concentration to obtain a new solution of different concentration
- 33. solute solute add water What happens to the amount of solute upon dilution ?
- 34. 1.8 moles 1.8 moles 6M HCl 300 mL. + 300 ml water 3M HCl 600 mL
- 35. IN DILUTION • : • Amount of solute in original solution = amount of solute in the prepared solution •
- 36. Calculate the M of 55.0 g NaCl in 125 ml of solution SOLUTIONso SOLUTE SOLVENT 55.0 g 125 ml M = wt/mwt/liter
- 37. Theory Acid Base Arrhenius One which contains a hydrogen which will be yielded as a H+1 in water One which contains a hydroxide which will be yielded as OH-! In water Bronstead Lowry Proton donor Proton acceptor Lewis E’ pair acceptor E’ pair donor
- 38. H Cl H +1 + Cl It has H +1: has a potential to donate Proton donor Bronsted acid H +1 E pair acceptor Lewis acid Arrhenius acid + 2e' H
- 39. Na OH OH -1 Na +1 + it has OH w/c is negative; potential proton acceptor Bronsted base OH -1 Na +1 + Has several e’ pairs; potential e’ pair donor Lewis base Arrhenius base
- 40. HCl H2O H + Cl - + Acid Conjugate base What had become of the acid after donating a proton
- 41. NH3 NH4 + H2O OH - BASE CONJUGATE ACID What had become of the base after donating 2 electrons
- 42. HCl H2O H + Cl - + acid Conjugate base base Conjugate acid A strong acid has a weak conjugate base SA Weak SB At equilibrium weak are favored
- 43. solute Solvent (water) solution electrolytes Strong : HCl H + Cl - + NaOH Na + OH - + Weak HC2H3O2 H + C2H3O2 - + Mg(O H) 2 Mg +2 2 OH - + Aside as being a solvent , something important is happening to water Acid, base, salt
- 44. H2O dissociates (into ions) to an extremely small but very important degree. Kw = 1 X 10 -14 (0.00000000000001) T= 250 C H2O H + OH - + Arithmetically of the value 1x10 -7 M 1x10 -7 M WATER IS A NON ELECTROLYTE Ion product constant : Kw = (H+) (OH-) Called acidity basicity neutral
- 45. solution • solute • Solvent (water) electrolyte Non electrolyte HCl H +1 Cl + NaOH OH - Na +1 + acid base HOHH + OH - + Interplay of these ions is ACIDITY AND OR BASICITY
- 47. When an acid is added to water , the H+ concentration of the resulting solution is determined solely by the acid base OH- base HCl Cl - H + 0.01 M H2O H + OH - H + solution = 0.01 0.01 NaOH Na + OH - + 0.02 M H2O H + OH - + OH- solution = 0.02 M 0.02 M Small amount acidic Small amount basic
- 48. Colligative Properties- dependent only on the ratio of the number of solute particle to the number of solvent particles and not on the nature of the solute. • Vapor pressure lowering • Boiling point elevation • Freezinf point Depression • Osmotic Pressure Does not matter wether solute is an acid or base or salt
- 49. Vapor pressure P air Temperature : Boiling point solvent
- 50. Vapor pressure P air Solute occupies some areas of the liquid and interfers with the evaporation less
- 51. Vapor pressure P air TBoiling point Elevation more
