Spectroscopy الشيث الرابع.pptx
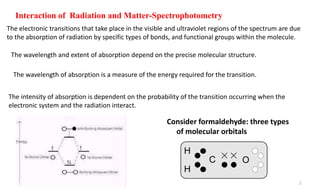
- 1. 1 Interaction of Radiation and Matter-Spectrophotometry The electronic transitions that take place in the visible and ultraviolet regions of the spectrum are due to the absorption of radiation by specific types of bonds, and functional groups within the molecule. The wavelength and extent of absorption depend on the precise molecular structure. The intensity of absorption is dependent on the probability of the transition occurring when the electronic system and the radiation interact. H H C O Consider formaldehyde: three types of molecular orbitals The wavelength of absorption is a measure of the energy required for the transition.
- 2. 2 Electronic Excitation The absorption of light energy by organic compounds in the visible and ultraviolet region involves the promotion of electrons in , , and n- orbitals from the ground state to higher energy states. This is also called energy transition. These higher energy states are molecular orbitals called antibonding. The higher energy transitions ( *) occur a shorter wavelength and the low energy transitions (*, n *) occur at longer wavelength. Energy * * n * * n * n * Antibonding Antibonding Nonbonding Bonding Bonding
- 3. The outer electrons in an organic molecule may occupy one of three different energy levels: 1- Sigma () electrons (single covalent bond (σ-bond): They are bonding electrons posses the lowest energy level ( it is the most stable). 2- Pi () electrons: They are bonding electrons of higher energy than sigma electrons. 3- Non-bonding (n) electrons: They are of atomic orbital of hetero atoms (N,O, halogen or S) which don’t participate in bonding, they usually occupy the highest level of ground state. In excited state: electrons under goes σ-σ* transition [high energy]. -electrons under goes -* transition, while, n electrons under goes n- * or n-* transitions. 3
- 4. Interaction of Radiation and Matter-Spectrophotometry Most σ-σ* absorption for individual bonds takes place below 200 nm in the vacuum UV-region and compound containing just σ-bonds are transparent in the UV-VIS region. π-π* and n- π* the absorption occurs in the UV- VIS region. π-π* and n- π* transitions involve important functional groups that are characteristic of many analyte. ground state excited state 4 Compounds containing only -electrons are the saturated hydrocarbons which absorbs <200nm (i.e.) in the far UV. They are transparent in the near UV (200-300nm) making them ideal solvents for other compounds to be studied in this region.
- 5. The absorbance of EMR in the UV-VIS regions depends on the structure of organic molecules. Absorbance of EMR by organic molecule is achieved by chromophoric groups (chromophores) assisted by auxochromes, where the electrons of absorbing molecule is excited, i.e it undergo transition from the ground state to the excited state. CHROMOPHORE: This term was previously used to denote a functional group of some other structural feature of which gives a color to compound. For example- Nitro group is a chromophore because its presence in a compound gives yellow color to the compound. But these days the term chromophore is used in a much broader sense which may be defined as “any group which exhibit absorption of electromagnetic radiation in a visible or ultra-visible region “It may or may not impart any color to the compound Chromophores are unsaturated groups responsible of π-π* and n-π* electronic transitions and imparts color to the molecules e.g C=C, C=O, N=N, N=O. AUXOCHROMES: It is a group which itself does not act as a chromophore but when attached to a chromophore, it shifts the absorption towards longer wavelength along with an increase in the intensity of absorption. Auxochromes act by entering into resonance interaction and shift the λmax to longer 5
- 6. 6 The absorbing groups in a molecule are called chromophores. Amolecule containing a chromophore is called a chromogen. An auxochrome does not itself absorb radiation, but, if present in a molecule, it can enhance the absorption by a chromophore
- 7. Interaction of Radiation and Matter-Spectrophotometry Bathochromic shift (Red-shift): is the shift of λmax to longer wavelength. Hypsochromic Shift (blue-shift): is the shift of λmax to shorter wavelength. Hyperchromic effect: is an increase in absorption intensity (absorptivity). Hypochromic effect is a decrease in absorption intensity (absorptivity). 7
- 8. Spectrophotometry Conjugation of double bonds lowers the energy required for the transition. In molecules containing a series of alternating double bonds (conjugated systems), the π-electrons are delocalized and require less energy for excitation, so that the absorption shifts to longer wavelengths. 8 1. Conjugation Effect Generally, extending conjugation leads to red shift
- 9. 2. Effect of pH The spectra of compounds containing acidic (phenolic-OH) or basic (-NH2) groups are dependent on the pH of the medium. 9 1. Phenol The spectrum of phenol in alkaline medium exhibits bathochromic shift (red shift), due to delocalization of electrons that required lower energy for excitation so appear at longer wavelength O O OH un-dissociated form Acid-medium dissociated form Alkaline-medium 2. Aniline The absorption spectrum of aniline in acid medium shows hypsochromic shift (blue-shift) and hypochromic effect. This blue-shift is due to the protonation of the amino-group, hence the pair of electrons is no longer available for the quinonoid conjugated structure which is formed in alkaline medium. NH3 NH2 NH2 Acid-medium Alkaline-medium
- 10. 2. Effect of pH When running the UV/VIS absorption spectra of a know concentration of phenol as a function of pH of the medium, all the spectra interact at certain wavelength which is called "The ISOBESTIC Point". 10 ISOBESTIC Point At this wavelength the two absorbing species, quinonoid and benzenoid for phenol, have the same asbsorptivity (absorbance). At isosbestic point, absorbance is not pH dependent.
- 11. 3. Dilution Effect An example of this effect is the change of color of dichromate solution upon dilution with water as given by the following equilibrium. 11 4. Solvent Effect The same substance usually has different spectrum in different solvent. The spectrum in polar solvent varies than in non-polar solvents. Polar solvent tend to interact with functional groups present in the molecules by hydrogen-bond and Vander- Wal-forces. Cr2O7 2- + H2O H2CrO4 2H+ + 2CrO4 2- Orange Ymax= 440 nm Yellow Ymax= 390 nm 5. Temperature Effect Change in temperature may shift the ionic equilibrium, so the temperature must be the same for all measurements.
- 12. Spectrophotometry Choice of solvent 12 The requirements for a solvent to be used in colorimetric or spectrophotometric determinations are: 1. must dissolve the substance under determination. 2. dose not interact with the solute. 3. must not show significant absorption at the wavelength to be employed in the determination. • Water is an excellent solvent, because it is transparent throughout the visible region and down to a wavelength of a bout 200 nm. •Aliphatic hydrocarbons, methanol, ethanol and diethyl ether are transparent to ultraviolet radiation and are frequently employed as solvents for organic compounds.
- 13. 13 Quantitative Applications The important characteristics of spectrophotometric and photometric methods are: 1- Wide applicability. 2- High sensitivity. 3- Moderate to high selectivity. 4- Good accuracy. 5- Ease and convenience. Application to Absorbing Species Spectrophotometric determination of organic compounds containing chromophores is thus potentially feasible. A number of inorganic species also absorb. We have noted that many ions of the transition metals are colored in solution and can thus be determined by spectrophotometric measurement. In addition, a number of other species show characteristic absorption peaks, including nitrite, nitrate, and chromate ions, the oxides of nitrogen, the elemental halogens, and ozone.
- 14. 14 Quantitative Applications Many non absorbing analytes can be determined photometrically by causing them to react with chromophoric reagents to give products that absorb strongly in the ultraviolet and visible regions. The successful application of these color- forming reagents usually requires that their reaction with the analyte be forced to near completion. Typical inorganic reagents include the following: thiocyanate ion for iron(III). Applications to Nonabsorbing Species :
- 15. 15 wave Length A 430 0.08 450 0.22 470 0.29 490 0.44 510 0.62 520 0.85 540 0.87 570 0.42 600 0.16 630 0.06
- 16. • Draw the calibration curve then determine the conc. of sample 1 and 2 Concentration Of standards Absorbance A ug/L * 0 0.00 2 0.13 4 0.25 6 0.38 8 0.52 10 0.64 12 0.77 Sample 1 0.34 Sample 2 0.68
- 17. • Draw the standard addition curve then determine the conc. of the unknown sample. Concentration Added to sample Absorbance A ug/L * 0 0.35 2 0.48 4 0.60 6 0.73 8 0.87 10 0.99