07 - PPT - reversible reactions NJT modified.pptx
•Descargar como PPTX, PDF•
0 recomendaciones•26 vistas
Introduction to Reversible Reactions
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Recomendados
Más contenido relacionado
Similar a 07 - PPT - reversible reactions NJT modified.pptx
Similar a 07 - PPT - reversible reactions NJT modified.pptx (20)
Chemistry 201 Laboratory- Harold Washington College Reac

Chemistry 201 Laboratory- Harold Washington College Reac
ENG 107 Commentary 1HW for Tu, April 19th – Commentary .docx

ENG 107 Commentary 1HW for Tu, April 19th – Commentary .docx
Último
Último (20)
All-domain Anomaly Resolution Office U.S. Department of Defense (U) Case: “Eg...

All-domain Anomaly Resolution Office U.S. Department of Defense (U) Case: “Eg...
Biopesticide (2).pptx .This slides helps to know the different types of biop...

Biopesticide (2).pptx .This slides helps to know the different types of biop...
High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...

High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...
SAMASTIPUR CALL GIRL 7857803690 LOW PRICE ESCORT SERVICE

SAMASTIPUR CALL GIRL 7857803690 LOW PRICE ESCORT SERVICE
Hubble Asteroid Hunter III. Physical properties of newly found asteroids

Hubble Asteroid Hunter III. Physical properties of newly found asteroids
Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b

Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b
PossibleEoarcheanRecordsoftheGeomagneticFieldPreservedintheIsuaSupracrustalBe...

PossibleEoarcheanRecordsoftheGeomagneticFieldPreservedintheIsuaSupracrustalBe...
Seismic Method Estimate velocity from seismic data.pptx

Seismic Method Estimate velocity from seismic data.pptx
Pulmonary drug delivery system M.pharm -2nd sem P'ceutics

Pulmonary drug delivery system M.pharm -2nd sem P'ceutics
Pests of mustard_Identification_Management_Dr.UPR.pdf

Pests of mustard_Identification_Management_Dr.UPR.pdf
Kochi ❤CALL GIRL 84099*07087 ❤CALL GIRLS IN Kochi ESCORT SERVICE❤CALL GIRL

Kochi ❤CALL GIRL 84099*07087 ❤CALL GIRLS IN Kochi ESCORT SERVICE❤CALL GIRL
07 - PPT - reversible reactions NJT modified.pptx
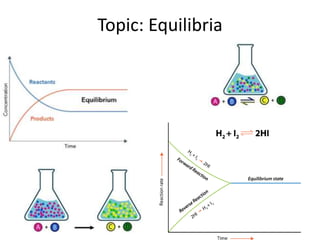
- 2. Discuss in your pairs and describe the above equation as much as you can on your mini WBs Starter • In the forward reaction A and B are the reactants and C and D are the products. • In the reverse reaction C and D are the reactants and A and B are the products. • The forward reaction is exothermic which means the reverse reaction is endothermic • The same amount of energy given out in the forward reaction is the same amount of energy taken in in the reverse reaction. Energy is given out
- 3. Practical CuSO4.5H2O CuSO4 + 5H2O hydrated copper sulfate (blue) anhydrous copper sulfate (white) Energy taken in Predict the following: What would you observe when the hydrated copper sulfate is heated? After heating the test tube is left to cool, what do you think will happen when a few drops of water is added to the white solid How do you think the test tube will feel to touch when water is added to the white solid?
- 4. Method • Add a spatula of hydrated copper sulfate (CuSO4.5H2O) into a boiling tube • Using tongs and holding the test tube away from your body heat on the Bunsen burner till all the solid turns white • Leave the boiling tube to cool. • Once the boiling tube has cooled down add a 2-3 drops of water using a dropper. • Carefully touch the boiling tube to determine if a temperature change has occurred See if your predictions are right!
- 5. Quick questions In your notebooks: 1. A reversible reaction takes in 50 kilojoules (KJ) of energy in the forward reaction. In this reaction A gets broken down in B and C. (a) Write an equation to show the reversible reaction (b)What can you say about the energy transfer in the reverse reaction?
- 6. 2. Blue cobalt chloride crystals turn pink when they become damp. The formula for the 2 forms can be written as CoCl2.2H2O and CoCl2.6H2O. (a) How many moles of water will combine with 1 mole of CoCl2.2H2O to form CoCl2.6H2O (b)Write a balanced equation for the reaction, which is reversible. (c) How can pick cobalt chloride crystals be changed back to blue cobalt chloride crystals? (d)What can this reaction be used to test for?
- 7. Learning objectives • understand that some reactions are reversible and are indicated by the symbol ⇌ in equations • describe reversible reactions such as the dehydration of hydrated copper(II) sulphate and the effect of heat on ammonium chloride