Organogram_EN_2016
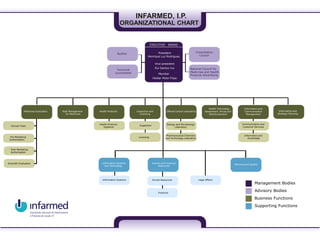
- 1. Business Functions Supporting Functions INFARMED, I.P. ORGANIZATIONAL CHART Management Bodies Information Systems and Technology Human and Financial Resources Planning and Quality Auditor EXECUTIVE BOARD Medicines Evaluation Health Products Inspection and Licensing Official Control Laboratory l Health Technology Assessment, Prices and Reimbursement Notified BodyRisK Management for Medicines Clinical Trials Scientific Evaluation Information Systems Biology and Microbiology Laboratory Consultative Council Pre-Marketing Authorisation Post-Marketing Authorisation National Council for Medicines and Health Products Advertising Communication and Customer Services Information and Knowledge Human Resources Financial InspectionHealth Products Vigilance Pharmaceutical Chemistry and Technology LaboratoryLicensing Information and Communication Management Information and Strategic Planning Legal Affairs Advisory Bodies EXECUTIVE BOARD President Member Rui Santos Ivo Vice-president Helder Mota Filipe Henrique Luz Rodrigues
