Bell 301 unit ii
- 1. BY PRASHANT KUMAR ASST. PROFESSOR MITS GWALIOR
- 3. The group of atoms is called the basis; when repeated in space it forms the crystal structure. The basis consists of a primitive cell, containing one single lattice point. Arranging one cell at each lattice point will fill up the entire crystal.
- 5. A crystal is one in which atoms or molecules are in three dimensional periodic arrangement. The periodicity may be same or different in different directions. The periodic positions of the atoms or molecules are called space lattice or crystal lattice. “The geometrical representation of a crystal structure in terms of lattice points is called space lattice”
- 6. The smallest portion of the crystal which can generate the complete crystal by repeating its own dimensions in various directions is called UNIT CELL
- 7. Axial lengths: a, b, c Interaxial angles : , ,
- 8. AUGUST BRAVAIS in 1848 mathematically proved that there are 14 distinct ways to arrange points in space.These 14 lattices corresponding to 7 crystal system are known as BRAVAIS LATTICE.
- 16. CONTRIBUTION by atoms at CORNER = 1/8 atom Contribution by atoms at Face center =1/2 atom Contribution by atoms at Body center= 1 atom Contribution by atoms at Base Center/End Center=1/2 atom NO. of faces = 6 NO. of corners=8 NO. of Bases =2 (top/bottom)
- 17. Type of unit cell No. of atoms At corners No. of atoms In faces No. of atoms At the center Total Simple Cubic 8 x 1/8 = 1 0 0 1 Body Centered Cubic(B.C.C) 8 x 1/8 = 1 0 1 2 Face Centered Cubic(F.C.C) 8 x 1/8 = 1 6 x 1/2 = 3 0 4 End Centred Cubic 8 x 1/8 = 1 2 x 1/2 = 1 0 2
- 20. Procedure for finding Miller Indices Step 1: Determine the intercepts of the plane along the axes X,Y and Z in terms of the lattice constants a,b and c. Step 2: Determine the reciprocals of these numbers.
- 21. Plane ABC has intercepts of 2 units along X-axis,3 units alongY-axis and 2 units along Z-axis. DETERMINATION OF ‘MILLER INDICES’ Step 1:The intercepts are 2,3 and 2 on the three axes. Step 2:The reciprocals are 1/2, 1/3 and 1/2. Step 3:The least common denominator is ‘6’. Multiplying each reciprocal by lcd, we get, 3,2 and 3. Step 4:Hence Miller indices for the plane ABC is (3 2 3)
- 22. Plane parallel toY and Z axes In this plane, the intercept along X axis is 1 unit. The plane is parallel toY and Z axes. So, the intercepts alongY and Z axes are . Now the intercepts are 1, and . The reciprocals of the intercepts are = 1/1, 1/ and 1/ . Therefore the Miller indices for the above plane is (1 0 0).
- 25. In such cases (when plane is passing through origin)the plane is translated by a unit distance along the non zero axis/axes and the Miller indices are computed 1. Intercept of shifted plan = ( 1 ) 2. Reciprocal 1 1 1 ( 1 ) Miller Indices (0 1 0)
- 38. BRAGG’S DIFFRACTION GRATING
- 40. • Why X-Ray is selected for crystallography ? Ans: because for proper observation of diffraction pattern Size of Obstacle (here it is Crystal) ~ should be comparable to wavelength of ray to be diffracted wavelength of X rays ~1Angstron Atomic distances in crystal~few angstron
- 41. Diffraction is the slight bending of light as it passes around the edge of an object. The amount of bending depends on the relative size of the wavelength of light to the size of the opening. If the opening is much larger than the light's wavelength, the bending will be almost unnoticeable.
- 46. DEFNITION Point defects,as the name implies, are imperfect point-like regions in the crystal.Typical size of a point defect is about 1-2 atomic diameters As the name suggests the size of the defect is very small. It is denoted by a point having no dimension. Dimension of point defects = 1-2 atomic diameters
- 47. A vacancy is a vacant lattice position from where the atom is missing. It is usually created when the solid is formed by cooling the liquid. There are other ways of making a vacancy, but they also occur naturally as a result of thermal excitation, and these are thermodynamically stable at temperatures greater than zero. At equilibrium, the fraction of lattice sites that are vacant at a given temperature (T) are So no of vacancy is dependent on temperature T exp o of vacancy of lattice point Q= constant T=temp in kelvin V L V L the energy required to move an atom from the interior of a crystal to its surface Q N N kT N n N k boltzmann no
- 48. An interstitial atom or interstitialcy is an atom that occupies a place outside the normal lattice position.It may be of the same type of atom as the rest surrounding it (self interstitial) or a foreign impurity atom. Interstitialcy is most probable if the atomic packing factor is low. Probablity of interstitialcy is highest in S.C > BCC > FCC (Bcz atomic packing factor of SC<BCC<FCC) Diameter of foreign atom < diameter of lattice atom/host atom Valency of foreign atom > valency of host atom Eg. Steel carbon occupies interstitial sites in Fe (iron) lattice increases strength of resulting material as number of bonds increases
- 49. Atom exchange with impurities Rate of substitution depends on i) Activation energy to exchange Condition for substitution Diameter of impurity/dopant ~ diameter of host atom Valency of impurity/dopant >/< vacancy of host atom Eg Doping in Semiconductor 1. Acceptor impurity doping in silicon by 13group elements (Al, Ga, In) 2. Donar impurity doping in Silicon by 15th group element (Phosphorus) • Eg.2 if we add copper to nickel, copper atoms will occupy crystallographic sites where nickel atoms would normally be present. • The substitutional atoms will often increase the strength of the metallic material.
- 50. In ionic crystals, existence of point defects is subjected to the condition of charge neutrality. There are two possibilities for point defects in ionic solids. - when an ion displaced from a regular position to an interstitial position creating a vacancy, the pair of vacancy-interstitial is called Frenkel defect. Cations are usually smaller and thus displaced easily than anions. Closed packed structures have fewer interstitials and displaced ions than vacancies because additional energy is required to force the atoms into the interstitial positions. - a pair of one cation and one anion can be missing from an ionic crystal, without violating the condition of charge neutrality when the valency of ions is equal.The pair of vacant sites, thus formed, is called Schottky defect.This type of point defect is dominant in alkali halides.These ion-pair vacancies, like single vacancies, facilitate atomic diffusion.
- 51. Frenkel Defects: some ions ,generally cations due to smaller size, move from regular lattice point to interstitial sites Schottky defects: When equal no of cations and anions are missing from regular lattice point. Both these defects maintain charge neutrality.
- 52. PARAMETERS SchottKy defects Frenkel defects REASON OF OCCURENCE Missing of equal no of cations & anions from lattice sites (Vacancy) due to missing of ions(generally cations) from lattice sites & these occupy Interstitial sites (Interstial) EFEECT ON DENSITY OF CRYSTAL DECREASES NO EFFECT GENERAL OCCURENCE FOUND IN STONG IONIC COMPOUNDS Eg. NaCl CaCl Found in crystal with Low coordination No. Eg. ZnS AgI Differences:
- 53. 1.Usually dislocations have a mixed character and Edge and Screw dislocations are the ideal extremes 2.Some types of dislocations can be visualized as being caused by the termination of a plane of atoms in the middle of a crystal. CAUSE OF DISLOCATIONS: 1. SHEAR STRESS
- 54. STEPS OF MODELLING SCREW DISLOCATIONS 1.TAKE A PERFECT CRYSTAL 2. CUT THE CRYSTAL HALF WAY 3.SKEW/SHEAR THE CRYSTAL BY ONE ATOM
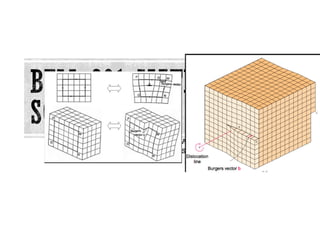
- 55. If we follow a crystallographic plane one revolution around the axis on which the crystal was skewed, starting at point x and traveling equal atom spacings in each direction, we finish at point y one atom spacing below our starting point. If a screw dislocation were not present, the loop would close. The vector required to complete the loop is the Burgers vector b. If we continued our rotation, we would trace out a spiral path. The axis, or line around which we trace out this path, is the screw dislocation. The Burgers vector is parallel to the screw dislocation.
- 58. Dislocation is the boundary between slipped and unslipped part of the crystal lying over slip plane
- 60. POSTIVE EDGE DISL. ( EXTRA HALF PLANE INSERTED IN UPPER HALF) NEGATIVE EDGE DISLOCATIONS (EXTRA HALF PLANE INSERTED IN LOWER HALF PLANE) STEPS TO MODEL EDGE DISLOCATION 1.TAKE PERFECT CRYSTAL 2.CUT CRYSTAL INTO TWO EQUAL HALVES 3.INSERT AN EXTRA HALF PLANE OF ATOMS
- 61. The bottom edge of this inserted plane represents the edge dislocation line. If we describe a clockwise loop around the edge dislocation,starting at point x and traveling an equal number of atom spacings in each direction,we finish at point y one atom spacing from the starting point. If an edge dislocation were not present, the loop would close.The vector required to complete the loop is, again,the Burgers vector (b). In this case, the Burgers vector is perpendicular to the dislocation. Top half plane of atoms=compression Bottom half plane of atoms=tension Burger vector is perpendicular to dislocation
- 64. The dislocation moves due to the change in the position of the top half of the atoms relative to the bottom half. Because the motion of the atoms results in thermodynamically stable states as the dislocation moves, the structural changes do not reverse when the shear stress is removed.To put it in continuum mechanics terms: we've plastically deformed our material because we moved the dislocation. This mechanism of plastic deformation by motion of dislocations is called slip
- 65. Non Stoichiometric defect: In non-Stoichiometric defect the ratio of number of cations and anions is disturbed by addition or removal of the ions. They are of two types: Metal deficiency defect: In this the solids have less number of metals relative to the described Stoichiometric proportion. Metal excess defect: There are two types of metal excess defect: Metal excess defect due to anionic vacancies:This kind of defect occurs due to absence of anions from its original lattice site in crystals.This position is occupied by electrons. Metal excess defect due to presence of extra cations at interstitial sites: In this type of defect extra cations are released by the compound on heating .This extra cation occupies the interstitial sites in crystals and the equal number of electrons goes to neighbouring interstitial sites.
- 66. 1 Dimensional Defect or Line defect Atoms surrounding the defects are Misaligned. EDGE DISLOCATION: 1.An edge dislocation is a defect where an extra half-plane of atoms is introduced midway through the crystal, distorting nearby planes of atoms. 2.MOVEMENT OF EDGE DISLOACTION:When enough force is applied from one side of the crystal structure, this extra Half plane passes through planes of atoms breaking and joining bonds with them until it reaches the grain boundary.
- 68. 1.External Surface: Surface atoms have unsatisfied atomic bonds, and higher surface energies, than the bulk atoms. To reduce surface free energy, material tends to minimize its surface areas against the surface tension (e.g. liquid drop). 2.Grain boundaries: regions between two adjacent grains slightly disordered low density in grain boundaries high mobility high diffusivity high chemical reactivity
- 69. 3.Tilt Boundary: A Tilt Boundary, between two slightly mis-aligned grains appears as an array of edge dislocations. Rotation axis is parallel to the boundary plane 4.Twist Boundary: Rotation axis is perpendicular to the boundary plane Low angle grain boundaries, that appear as an array of Screw dislocations.
- 70. 5.Twin boundaries: These are the boundaries in the grains at which the atomic arrangement on one side of the boundary is the mirror image of the atoms on the other side . May be produced by shear deformation. The region between the pair of boundaries is called the twinned region.
- 71. 6.Stacking Faults: Formed by fault in the stacking sequence of atomic planes in crystals. Considering stacking arrangement in FCC: (ABC sequence repeats) ABCABCABCABCABC ABCABC BCABCABC This thin region is a surface imperfection and is called Stacking faults.
- 72. Volume defects in crystals are three-dimensional aggregates of atoms or vacancies. It is common to divide them into four classes in an imprecise classification that is based on a combination of the size and effect of the particle. The four categories are: precipitates: which are a fraction of a micron in size and decorate the crystal; dispersant: which vary in size from a fraction of a micron to the normal grain size (10-100µm), but are intentionally introduced into the microstructure; Inclusions: vary in size from a few microns to macroscopic dimensions, and are relatively large, undesirable particles that entered the system as dirt or formed by precipitation; voids, which are holes in the solid formed by trapped gases or by the accumulation of vacancies.
- 73. Laue method Rotating crystal method Powder method METHOD LAUE METHOD Variable Fixed ROTATING CRYSTAL METHOD Fixed Variable Powder method Fixed Variable 2 sinn d What are the ways in which we can use Bragg’s law ? Thus, there are two handles that we can vary - λ and θ. Based on these handles there are essentially 3 methods of doing diffraction,as tabulated in 1 The difference between the Rotating crystal and Powder method is in how the angle is varied.
- 74. X RAY DIFFRACTION TECHNIQUES LAUE METHOD Used to find crystal ORIENTATION SINGLE CRYSTAL FIXED ANGLE POLYCHROMATIC BEAM (wavelength varied) ROTATING CRYSTAL TYPE Used to find LATICE CONSTANT SINGLE CRYSTAL VARIABLE ANGLE (varied using rotation) MONOCHROMATIC BEAM POWDER METHOD Used to find Lattice parameters Polycrystal Monochromatic beam Variable angle (varied using orientation)
- 75. Laue method was the first diffraction method used. A single crystal(fixed orientation) is required for this method. The technique use white radiation (range of wavelengths), typically unfiltered radiation from a X-ray tube.This falls on a single crystal that is fixed. Thus, the diffraction angle,θ, is fixed for every diffraction plane and for that particular θ value a certain wavelength will get diffracted, using Bragg’s equation .Thus, each diffracted beam will correspond to a certain wavelength which then falls on the film. Based on how the film is positioned there are two types in Laue method
- 76. • Used to find LATICE CONSTANT (d) • SINGLE CRYSTAL • VARIABLE ANGLE (varied using rotation) • MONOCHROMATIC BEAM In the rotating crystal method, a single crystal is mounted with an axis normal to a monochromatic x-ray beam. A cylindrical film is placed around it & the crystal is rotated about the chosen axis. • As the crystal rotates,Sets of lattice planes will at some point make the correct Bragg angle for the monochromatic incident beam, & at that point a diffracted beam will be formed. 2 sind n
- 77. If a powdered specimen is used, instead of a single crystal, then there is no need to rotate the specimen, because there will always be some crystals at an orientation for which diffraction is permitted. Here a monochromatic X-ray beam is incident on a powdered or polycrystalline sample. This method is useful for samples that are difficult to obtain in single crystal form
- 78. The powder method is used to determine the value of the lattice parameters accurately. Lattice parameters are the magnitudes of the unit vectors a, b and c which define the unit cell for the crystal. For every set of crystal planes, by chance, one or more crystals will be in the correct orientation to give the correct Bragg angle to satisfy Bragg's equation. Every crystal plane is thus capable of diffraction. Each diffraction line is made up of a large number of small spots, each from a separate crystal. Each spot is so small as to give the appearance of a continuous line.
- 79. If a monochromatic X-ray beam is directed at a single crystal, then only one or two diffracted beams may result. See figure For a sample of several randomly orientated single crystals, the diffracted beams will lie on the surface of several cones.The cones may emerge in all directions, forwards and backwards. See figure For a sample of hundreds of crystals (powdered sample), the diffracted beams form continuous cones. A circle of film is used to record the diffraction pattern as shown. Each cone intersects the film giving diffraction lines.The lines are seen as arcs on the film. See figure The Powder Method 79
- 80. ADVANTAGES & DISADVANTAGES OF XRD COMPARED TO OTHER METHODS Advantages X-Rays are the least expensive, the most convenient & the most widely used method to determine crystal structures. X-Rays are not absorbed very much by air, so the sample need not be in an evacuated chamber. Disadvantages X-Rays do not interact very strongly with lighter elements.
- 83. [1] http://nptel.ac.in/courses/113106032/6%20-%20Lattice%20defects.pdf [2] http://nptel.ac.in/courses/113105023/Lecture10.pdf [3] http://nptel.ac.in/courses/Webcourse-contents/IISc- BANG/Material%20Science/pdf/Lecture_Notes/MLN_03.pdf [4] https://sparkyswordscience.blogspot.in/2013/12/introduction-to-crystal- structure.html
- 84. characterization of crystalline materials identification of fine-grained minerals such as clays and mixed layer clays that are difficult to determine optically determination of unit cell dimensions measurement of sample purity
