Jurisprudence With Pharmacy Act (State or Centre).pdf
•
2 recomendaciones•844 vistas
urisprudence is the study of the science of law. The study of law in jurisprudence is not about any particular statute or a rule but of law in general, its concepts, its principles and the philosophies underpinning it. The primary object of the interpretation is to discover the true intention of the Legislature
Denunciar
Compartir
Denunciar
Compartir
Descargar para leer sin conexión
Recomendados
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
Medicinal and toilet preparations act and rules,1955

Medicinal and toilet preparations act and rules,1955
Factors affecting protein drug binding and its kinetics

Factors affecting protein drug binding and its kinetics
P'JURISPRUDENCE SCHEDULES IN DETAIL RGPV Unit 2 d&c act part 1

P'JURISPRUDENCE SCHEDULES IN DETAIL RGPV Unit 2 d&c act part 1
Drugs and Magic Remedies (Objectionable Advertisements), 1954 Act,1954 

Drugs and Magic Remedies (Objectionable Advertisements), 1954 Act,1954
Similar a Jurisprudence With Pharmacy Act (State or Centre).pdf
Similar a Jurisprudence With Pharmacy Act (State or Centre).pdf (20)
Pharmacy act - 1948 sem.4 b.pharm pharm.jurisprudense

Pharmacy act - 1948 sem.4 b.pharm pharm.jurisprudense
IIMPORT AND REGISTRATION AS PER DRUG AND COSMETIC ACT 

IIMPORT AND REGISTRATION AS PER DRUG AND COSMETIC ACT
Drugscosmeticsact1940 120826115235-phpapp02.pptx-1

Drugscosmeticsact1940 120826115235-phpapp02.pptx-1
Más de RAHUL PAL
Más de RAHUL PAL (20)
Software Used In Formulation Design Process- Minor Project [Bachelor].pdf![Software Used In Formulation Design Process- Minor Project [Bachelor].pdf](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Software Used In Formulation Design Process- Minor Project [Bachelor].pdf](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Software Used In Formulation Design Process- Minor Project [Bachelor].pdf
𝐎𝐫𝐚𝐥 𝐏𝐚𝐩𝐞𝐫 𝐏𝐫𝐞𝐬𝐞𝐧𝐭𝐚𝐭𝐢𝐨𝐧: 𝐈𝐧𝐭𝐞𝐫𝐧𝐚𝐭𝐢𝐨𝐧𝐚𝐥 𝐂𝐨𝐧𝐟𝐞𝐫𝐞𝐧𝐜𝐞 (𝐈𝐑𝐓𝐄𝐂 𝟐.𝟎-𝟐𝟎𝟐𝟒); The Curre...

𝐎𝐫𝐚𝐥 𝐏𝐚𝐩𝐞𝐫 𝐏𝐫𝐞𝐬𝐞𝐧𝐭𝐚𝐭𝐢𝐨𝐧: 𝐈𝐧𝐭𝐞𝐫𝐧𝐚𝐭𝐢𝐨𝐧𝐚𝐥 𝐂𝐨𝐧𝐟𝐞𝐫𝐞𝐧𝐜𝐞 (𝐈𝐑𝐓𝐄𝐂 𝟐.𝟎-𝟐𝟎𝟐𝟒); The Curre...
THE CURRENT STATUS IN MUCOSAL DRUG DELIVERY SYSTEM (MDDS) AND FUTURE PROSPECT...

THE CURRENT STATUS IN MUCOSAL DRUG DELIVERY SYSTEM (MDDS) AND FUTURE PROSPECT...
Design of Experiments (DoE) manipulation in the formulation and optimization ...

Design of Experiments (DoE) manipulation in the formulation and optimization ...
The Utilization of 32 Full Factorial Design (FFD) for Optimization of Linco...

The Utilization of 32 Full Factorial Design (FFD) for Optimization of Linco...
Determination of Partition coefficient of Known and Unknown drug.pdf

Determination of Partition coefficient of Known and Unknown drug.pdf
Partition Coefficient Determination (Pharmaceutics Practical).pptx

Partition Coefficient Determination (Pharmaceutics Practical).pptx
Research Methodology_UNIT_V_Declaration of Helsinki M. Pharm (IIIrd Sem.)

Research Methodology_UNIT_V_Declaration of Helsinki M. Pharm (IIIrd Sem.)
The Utilization of Response Surface Methodology (RSM) In the Optimization of ...

The Utilization of Response Surface Methodology (RSM) In the Optimization of ...
Research Methodology (M. Pharm, IIIrd Sem.)_UNIT_IV_CPCSEA Guidelines for Lab...

Research Methodology (M. Pharm, IIIrd Sem.)_UNIT_IV_CPCSEA Guidelines for Lab...
MEDICAL RESEARCH: UNIT_III_ EUTHANASIA, COI, CONFIDENTIALITY RESEARCH METHODO...

MEDICAL RESEARCH: UNIT_III_ EUTHANASIA, COI, CONFIDENTIALITY RESEARCH METHODO...
Biostatistics_Unit_II_Research Methodology & Biostatistics_M. Pharm (Pharmace...

Biostatistics_Unit_II_Research Methodology & Biostatistics_M. Pharm (Pharmace...
(I) MEDICAL RESEARCH_ UNIT_III_RESEARCH METHODOLOGY & BIOSTATISTICS.pptx

(I) MEDICAL RESEARCH_ UNIT_III_RESEARCH METHODOLOGY & BIOSTATISTICS.pptx
Research Article Published: "Optimization and formulation of dox loaded lipos...

Research Article Published: "Optimization and formulation of dox loaded lipos...
Último
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Último (20)
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
Z Score,T Score, Percential Rank and Box Plot Graph

Z Score,T Score, Percential Rank and Box Plot Graph
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes

Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Jurisprudence With Pharmacy Act (State or Centre).pdf
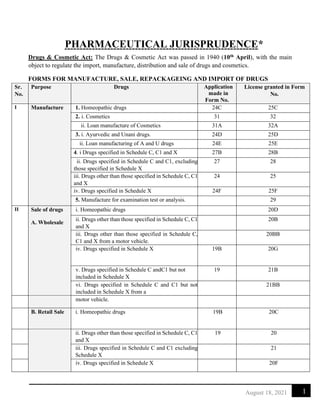
- 1. 1 August 18, 2021 PHARMACEUTICAL JURISPRUDENCE* Drugs & Cosmetic Act: The Drugs & Cosmetic Act was passed in 1940 (10th April), with the main object to regulate the import, manufacture, distribution and sale of drugs and cosmetics. FORMS FOR MANUFACTURE, SALE, REPACKAGEING AND IMPORT OF DRUGS Sr. No. Purpose Drugs Application made in Form No. License granted in Form No. I Manufacture 1. Homeopathic drugs 24C 25C 2. i. Cosmetics 31 32 ii. Loan manufacture of Cosmetics 31A 32A 3. i. Ayurvedic and Unani drugs. 24D 25D ii. Loan manufacturing of A and U drugs 24E 25E 4. i Drugs specified in Schedule C, C1 and X 27B 28B ii. Drugs specified in Schedule C and C1, excluding those specified in Schedule X 27 28 iii. Drugs other than those specified in Schedule C, C1 and X 24 25 iv. Drugs specified in Schedule X 24F 25F 5. Manufacture for examination test or analysis. 29 II Sale of drugs A. Wholesale i. Homeopathic drugs 20D ii. Drugs other than those specified in Schedule C, C1 and X 20B iii. Drugs other than those specified in Schedule C, C1 and X from a motor vehicle. 20BB iv. Drugs specified in Schedule X 19B 20G v. Drugs specified in Schedule C andC1 but not included in Schedule X 19 21B vi. Drugs specified in Schedule C and C1 but not included in Schedule X from a 21BB motor vehicle. B. Retail Sale i. Homeopathic drugs 19B 20C ii. Drugs other than those specified in Schedule C, C1 and X 19 20 iii. Drugs specified in Schedule C and C1 excluding Schedule X 21 iv. Drugs specified in Schedule X 20F
- 2. 2 August 18, 2021 (C) Restricted i. Drugs other than those specified in Schedule C, C1 and X 20A ii. Drugs specified in Schedule C, C1 but not in Schedule X 21A III Repackaging Drugs specified in Schedule C and C1 24B 25B IV Import i. Drugs specified in Schedule C and C1 8 10 ii. Drugs specified in Schedule X 8a 10a iii. Import of drugs for testing 11 iv. Small quantity of drugs for personal use 12 12B PHARMACYACT* The Pharmacy Council of India is made up of the following members: A. Elected members: i. Six members, among whom at least one is a teacher of Pharmaceutical Chemistry, Pharmacy, Pharmacology and Pharmacognosy of an Indian University, or a college affiliated thereto, which grants a degree or diploma in Pharmacy, elected by the University Grants Commission. ii. One member elected from amongst themselves by the Members of the Medical Council of India. iii. One member to represent each State, nominated by State Government, who shall be a Registered Pharmacist. B. Nominated members: i. Six members, four of whom are persons possessing a degree or diploma in, and practicing, pharmacy or pharmaceutical chemistry, nominated by the Central Government. ii. A representative of the University Grants Commission and a representative of the All-India Council for Technical Education. iii. One member to represent each State elected [from amongst themselves] by the members of each State Council, who shall be a Registered Pharmacist. C. Ex-officio members i. Director General of Health Services, or a person authorized by him. ii. Drugs Controller of India or a person authorized by him. iii. Director of Central Drugs Laboratory.
- 3. 3 August 18, 2021 STATE PHARMACY COUNCIL State Pharmacy Council and Joint State Pharmacy Council are also constituted by State Governments. Two or more states may also agree that the State Council of one state shall serve the, needs of the other participating States. Two or more States may also enter into agreement (inter-state agreement) for definite specified period, to form Joint State Council. Pharmacy Council and Joint State Pharmacy Council, as follows- State Council Joint Council 1. Six Registered Pharmacists elected from amongst themselves. 2. One member elected from amongst themselves by the Members of the Medical Council of the State. Nominated Members 1. Five members, of whom at least three shall possess a degree or diploma in Pharmacy or Pharmaceutical Chemistry, or be Registered Pharmacists, nominated by the State Government. Ex-officio Members: 1. Chief of Administrative Medical Officer of the state. 2. Officer–in-charge of Drugs and Cosmetics Act, 1940. 3. Government Analyst under the Drugs and Cosmetics Act, or where there is more than one, such as the State Government may appoint in this behalf. 1. 3-5 Registered Pharmacists of each of participating State, elected from amongst themselves. However, if agreed, the number of members elected by each of the participating state may not be the same, but shall vary from 3-5. 2. One member elected by Medical Council of each State from amongst its members. 1. 2-4 members nominated by each participating State of whom more than half shall possess a degree or diploma in Pharmacy or Pharmaceutical Chemistry, or be Registered Pharmacists. However, if agreed, the number of members nominated by each of the participating State may not be the same, but shall be 2-4 only. 1. Chief of Administrative Medical Officer of each of the participating states. 2. Officer–in-charge of the Drugs Control Organization of each participating state. 3. Government Analyst of each participating state under the Drugs and Cosmetics Act, or where there is more than one in each state, such one as the State Government may appoint in his behalf.
