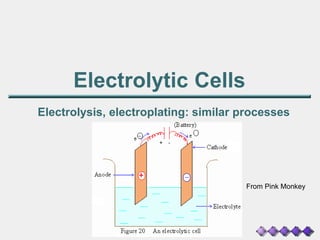
Electrolytic cells
- 1. Electrolytic Cells Electrolysis, electroplating: similar processes From Pink Monkey
- 2. Electrochemistry The Two Types ▪ Electrochemical Cells: ▸ Spontaneous flow of electrons from anode through a meter or “work” to the cathode. Energy is produced by the cell and used to do work ▪ Electrolytic Cells: ▸ Forced flow of electrons from anode to the cathode by an external battery or generator. Energy is used by the electrochemical cell to make high- energy elements or compounds
- 3. Example The Lead-Acid Battery (Pb/H2SO4/PbO2) ▪ Charged battery: Pb in contact with H2SO4: Pb oxidized to form PbSO4. This oxidation generates electrons (cathode) PbO2 in contact with H2SO4: PbO2 is reduced by adding electrons to form PbSO4. Discharged battery: PbSO4 coats both electrodes, surrounded by depleted sulfuric acid
- 4. Example The Lead-Acid Battery (Pb/H2SO4/PbO2) ▪ Discharged battery is recharged: PbSO4 coats both electrodes, surrounded by depleted sulfuric acid ▸ Electrons are forced in the opposite direction by the automobile generator PbSO4 is oxidized to PbO2 (+2 to +4) PbSO4 is reduced to Pb at the other electrode Now the Pb and PbO2 are restored at their proper electrodes so they can start your car the next time you need some “juice”
- 5. Summary Lead-Acid Battery ▪ Discharging: producing of electrons Pb + H2SO4 → PbSO4 + 2H+ + 2e- PbO2 + 4H+ + SO4 2- + 2e- → PbSO4 + 2H2O ▪ Charging: restoring original situation PbSO4 + 2H+ + 2e- → Pb + H2SO4 PbSO4 + 2H2O → PbO2 + 4H+ + SO4 2- + 2e- Batteries “die” when enough PbSO4 falls off one or the other electrode so that it can’t “hold a charge” in the electrolysis cycle
- 6. What Reaction Happens? If a voltage is supplied to a mixture of cations, which one will plate out? Since this would cause reduction, look at reduction potentials Ag+ + e- → Ag (s) E° = 0.80 V Cu2+ + 2e- → 2Cu (s) E° = 0.34 V Zn2+ + 2e- → 2Zn (s) E° = -0.76 V The more positive voltage has a more negative G Thus Ag+ >Cu2+ >Zn2+
- 7. Chlorine & NaOH Electrolysis of Brine (concentrated NaCl) ▪ Cathode: reduction--electrons provided 2 H2O + 2e- → H2 (g) + 2 OH- (aq) ▪ Anode: oxidation--electrons taken away 2 Cl- (aq) → Cl2 (g) + 2e- ▪ Valuable stuff produced: ▸ Hydrogen gas ▸ Sodium hydroxide (lye) ▸ Chlorine gas
- 8. Hydrogen & Oxygen Electrolysis of dilute sulfuric acid ▪ Cathode: reduction--electrons provided 2 H2O + 2e- → H2 (g) + 2 OH- (aq) ▪ Anode: oxidation--electrons taken away 2 H2O → O2 (g) + 4 H-(aq) + 4e- ▪ Valuable stuff produced: ▸ Hydrogen gas ▸ Oxygen gas ▪ Oxygen is more cheaply produced by liquefying air
- 9. Downs Cell Electrolysis of molten sodium chloride (m.p. = 1074K) From voltaicpower.com
- 10. Sodium & Chlorine Electrolysis of molten sodium chloride (m.p. = 1074K) ▪ Anode: oxidation 2 Cl- (l) → Cl2 (g) + 2e- ▪ Cathode: reduction 2 Na+ (l) + 2e- → 2 Na (l) ▪ This process can also be used to produce K from KCl, Li from LiCl, etc.
- 11. Electroplating Commercial use for electrolysis ▪ Silver plating Ag+ + e- → Ag (s) ▸ Should we put the item to be plated at – the cathode or the anode? ▪ Electrorefining: ▸ Impure metal at the anode; pure metal appears at the cathode ▪ How would we produce the selectively plated Toyota logo at top right?
- 12. Electrolysis Stoichiometry Commercial use for electrolysis We can predict amount of product at either electrode Current x time = coulombs of charge Coulombs x 1 mol e- = mol electrons 96,485 Coulombs Moles product = mol e- x Stoichiometric ratio Grams product = mol product x molar mass 1 mol electrons = 96,485 C = 1 Faraday
- 13. Electrolysis Stoichiometry Problem to work Current x time = coulombs of charge Coulombs x 1 mol e- = mol electrons 96,485 Coulombs Mol product = mol e- x Stoichiometric ratio Grams product = mol product x molar mass How much silver can we plate out with a 12.0 A current running 3.5 hours? Coulombs = 12.0 A x 3.5 hr x 60 min/hr = 2520 C Mol e- = 2520 C x 1 mol e- = 2.6 x 10-2 mol e- 96,485 C Mol Ag = 2.6 x 10-2 mol e- x 1 mol Ag = 2.6 x 10-2 mol Ag 1 mol e- Mass Ag = 2.6 x 10-2 mol Ag x 107.9 g Ag 1 mol Ag = 2.8 g Ag