How cells read the genome from DNA to protein Notes
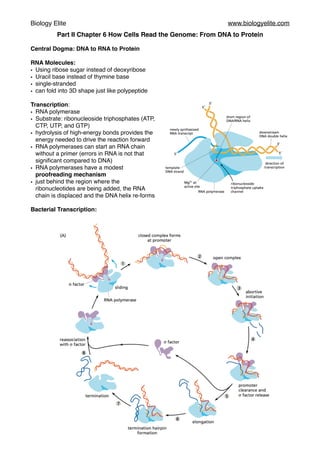
- 1. Biology Elite www.biologyelite.com Part II Chapter 6 How Cells Read the Genome: From DNA to Protein Central Dogma: DNA to RNA to Protein RNA Molecules: • Using ribose sugar instead of deoxyribose • Uracil base instead of thymine base • single-stranded • can fold into 3D shape just like polypeptide Transcription: • RNA polymerase • Substrate: ribonucleoside triphosphates (ATP, CTP, UTP, and GTP) • hydrolysis of high-energy bonds provides the energy needed to drive the reaction forward • RNA polymerases can start an RNA chain without a primer (errors in RNA is not that significant compared to DNA) • RNA polymerases have a modest proofreading mechanism • just behind the region where the ribonucleotides are being added, the RNA chain is displaced and the DNA helix re-forms Bacterial Transcription:
- 2. Biology Elite www.biologyelite.com • sigma (σ) factor associates with the core enzyme and assists it in reading the signals in the DNA that tell it where to begin transcribing • σ factor and core enzyme are known as the RNA polymerase holoenzyme • holoenzyme slide along the DNA sequence to find a starting signal called promoter • σ factor then binds tightly to the promoter • holoenzyme opens up the double helix and the core enzyme starts to transcribe RNA sequence • However, holoenzyme is still connected to the promoter, so the short RNAs are often released, forcing the core enzyme to start over. • Eventually this process of abortive initiation is overcome, and core enzymes discard the σ factor as well as the connection to promoter • Polymerase continue to transcribe in a stepwise fashion • The enzyme encounters a terminal signal called terminator • Terminator is a DNA sequence which consists of A-T nucleotide pairs, when the terminator is transcribe into RNA, the RNA will automatically folded into the hairpin shape, thereby stop the polymerase and release it from the DNA sequence • Core enzyme is released and reassociate with σ factor to form a holoenzyme Eukaryotic Transcription: • RNA polymerases I and III transcribe the genes encoding transfer RNA, ribosomal RNA, and various small RNAs. RNA polymerase II transcribes most genes, including all those that encode proteins, and our subsequent discussion therefore focuses on this enzyme. • Bacterial RNA polymerase only needs a single transcription factor: σ factor; while eukaryotic RNA polymerase requires many factors, collectively called general transcription factor. • Eukaryotic transcription must take place on DNA that is packed into nucleosomes and high- order forms of chromatin structure. • General transcription factor are needed for all promoters that RNA polymerase II uses. They are used to imitate the transcription and release the polymerase form the promoter to start transcribing.They are: TFIIA, TFIIB, TFIIC, TFIID, and so on (TFII standing for transcription factor for polymerase II). • TFIID binds to a short DNA sequence composed of the A and T, which is called a TATA box. The subunit of TFIID called TBP (TATA-binding protein) recognise the TATA box and binds to it. • The binding of TATA box causes the distortion in the DNA, it brings the DNA sequence on the both side together and creates a physical landscape of the promoter. • Other factors then assemble, along with the RNA polymerase II, to form a complete transcription initiation complex. • TFIIH, which contains DNA helicase as one of its subunits, unwind the DNA double helix, exposing the template strand. • Like bacterial RNA polymerase, RNA polymerase II also synthesises a short length of RNA and performs conformational changes before releasing form the promoter and start transcribing. • In this process, phosphate group is added to the tails of RNA polymerase II (known as the CTD or C-terminal domain) • In human, CTD contains 52 tandem repeats of a seven-amino acid sequence, serine located at the fifth position in the repeat sequence (Ser5) is phosphorylated by TFIIH • RNA polymerase II will then disengage from the general transcription factor. • In addition, transcriptional activators must bind to specific sequences in DNA (called enhancers) and help to attract RNA polymerase II to the start point of transcription
- 3. Biology Elite www.biologyelite.com • eukaryotic transcription initiation in vivo requires the presence of a large protein complex known as Mediator, which allows the activator proteins to communicate properly with the polymerase II and with the general transcription factors. • transcription initiation in a eukaryotic cell typically requires the recruitment of chromatin- modifying enzymes, including chromatin remodeling complexes and histone-modifying enzymes, which will increase access to the DNA in chromatin • RNA polymerase also causes the problem of DNA supercoiling: In eukaryotes, DNA topoisomerase keeps removing the supercoiling tension; in bacteria, DNA gyrase uses the energy of ATP to pump the supercoil into DNA Modification of eukaryotic pre-mRNA: • RNA 5’ end capping: one (a phosphatase) removes a phosphate from the 5ʹ end of the nascent RNA, another (a guanyl transferase) adds a GMP in a reverse linkage (5ʹ to 5ʹ instead of 5ʹ to 3ʹ), and a third (a methyl transferase) adds a methyl group to the guanosine
- 4. Biology Elite www.biologyelite.com • RNA cap helps the cell to distinguish mRNAs from the other types of RNA molecules present in the cell. RNA splicing: • Each splicing event removes one intron, proceeding through two sequential phosphoryl- transfer reactions known as transesterifications; these join two exons together while removing the intron between them as a “lariat” • Splice site contains a consensus short nucleotide sequence, which acts as a cue for where splicing is to take place. • RNA splicing is performed by spliceosome. 5 short RNA molecule: U1,U2,U4,U5 and U6,known as snRNA (small nuclear RNA), each is then complexed with protein subunits to form an snRNP (small nuclear ribonucleoprotein). snRNPs then form the core of spliceosome. • Recognition of splice junction is performed through base pairing between the snRNAs and the consensus RNA sequences.
- 5. Biology Elite www.biologyelite.com • Energy form ATP hydrolysis is used to build up the spliceosomes and break or form the base pairs between RNA subunits and/or mRNA. Fidelity of RNA splicing: • RNA polymerase tail carries several components of spliceosome, and these components are transferred directly from the polymerase to the RNA as the RNA emerges from the polymerase. This helps the cell to keep track of introns and exons. It helps the cell to mark the introns. • Exon definition: Exon size tends to be much more uniform, averaging about 150 nucleotide pairs. SR proteins assemble on exon sequence and help to mark off splice site and recruit protein, U1 for downstream and U2 for upstream. SR proteins bind preferentially to specific RNA sequence in eons, termed splicing enhancers. • Chromatin structure can alter the speed of transcribing thus splicing. So the speed can be minimised so exon skipping can be minimised as well. • Some histone modifications attract components of spliceosome, which will be transferred to emerging RNA.
- 6. Biology Elite www.biologyelite.com 3’ end process of RNA: • 3’ end of each mRNA molecule is specified by signals encoded in the genome. • Two multisubunit proteins, called CstF (cleavage stimulation factor) and CPSF (cleavage and polyadenylation specificity factor), are on the RNA polymerase tail.They binds to their recognition sequences on the emerging RNA molecule. • RNA is cleaved from the polymerase. An enzyme called poly-A polymerase (PAP) adds and produces around 200 A nucleotides to the 3’ end. • Poly-A-binding proteins assemble onto the A tail and help to determine the final length of the tail. • After the 3’ end of pre-mRNA has been cleaved, RNA polymerase II continues to transcribes. This unprotected RNA is then degraded by a exonuclease on the polymerase tail, which will eventually cause the termination of transcription. Export of mature RNA: • mRNA is distinguished by the protein it has. Cap-binding protein, exon junction complexes and poly-A-binding protein marks the completion of capping, splicing and poly-A addition. • Improperly processed mRNAs and other RNA debris (excised intron sequence) is processed and recycled by nuclear exosome, which is rich of exonuclease. • Successfully processed mRNAs are guided through the nuclear pore complexes.(NPCs) • RNA must pass through the pore by active transport. Nuclear transport receptor must be loaded onto RNA, which is performed with 3’ cleavage and polyadenylation. • Some protein attached on the RNA will travel out of the nucleus and some protein will remain attached. In addition, some protein will add onto the mature RNA molecule.
- 7. Biology Elite www.biologyelite.com Non-coding RNA: • ribosomal RNAs (rRNA) is the most abundant RNA in living organism. RNA polymerase I is dedicated to producing rRNAs. RNA polymerase I is absent of C-terminal, which explains why rRNA is neither capped or polyadenylated. • 4 types of rRNAs, 3 (18S, 5.8S, 28S) are made by chemically modifying and cleaving a single large precursor rRNA., fourth(5S) is synthesised from a separate cluster of genes by RNA polymerase III. • There are chemically modification(2’-O-methylated nucleotide, isomers of uridine called pseudouridine) on precursor rRNA before rRNAs are cleaved out and assemble into ribosomes. The modification is hypothesised to aid the folding and assembly of the final rRNA. • Nucleolus is the location where rRNAs are assembled to make ribosome as well as telomerase, tRNAs and other RNA-protein complexes. From RNA to Protein: • RNA sequence is divided into a group of three consecutive nucleotides, called codons. One amino acid can be coded with more than one codon. • tRNAs are responsible for carrying amino acids. They contain anticodons which can be paired with the codons on RNA. They are more than one type of tRNAs for an amino acid. Also, mismatch(wobble) is allowed for the third base pairing. So a tRNA can pair with more than one codon. • tRNA are synthesised by RNA polymerase III. They are covalently modified before exiting form the nucleus. • tRNA splicing uses a cut-and-paste mechanism rather than a lariat intermediate. Modified nucleotide sequence is also found in tRNA, which affects the conformation and base-paring of tRNA and thereby facilitate the recognition of the appropriate mRNA codons. • Recognition and attachment of the correct amino acid depends on enzymes called aminoacyl- tRNA synthetase. Most cell have different synthethase enzymes for different amino acids.In bacteria, less than 20 synthetase can be found, which means it can put identical amino acids on two types of tRNA. One is correct and another needs modification to become correct.
- 8. Biology Elite www.biologyelite.com • Most synthetase enzyme choose the correct amino acids in two steps: 1.The correct amino acid has the highest affinity for the active-site pocket of its synthetase and is therefore favored over the other 19 2.A second discrimination step occurs after the amino acid has been covalently linked to AMP: when tRNA binds, the synthetase tries to force the adenylated amino acid into a second editing pocket in the enzyme • Most tRNA synthetases directly recognize the matching tRNA anticodon; these synthetases contain three adjacent nucleotide-binding pockets, each of which is complementary in shape and charge to a nucleotide in the anticodon. RNA messages are decoded in ribosome
- 9. Biology Elite www.biologyelite.com • The small subunit provides the framework on which the tRNAs are accurately matched to the codons of the mRNA, while the large subunit catalyzes the formation of the peptide bonds that link the amino acids together into a polypeptide chain • a ribosome contains four binding sites for RNA molecules: one is for the mRNA and three (called the A site, the P site, and the E site) are for tRNAs • tRNA binding (step 1), peptide bond formation (step 2), large subunit translocation (step 3), and small subunit translocation (step 4). As a result of the two translocation steps, the entire ribosome moves three nucleotides along the mRNA and is positioned to start the next cycle. • In step 1, a tRNA carrying the next amino acid in the chain binds to the ribosomal A site by forming base pairs with the mRNA codon positioned there, so that the P site and the A site contain adjacent bound tRNAs. • In step 2, the carboxyl end of the polypeptide chain is released from the tRNA at the P site (by breakage of the high-energy bond between the tRNA and its amino acid) and joined to the free amino group of the amino acid linked to the tRNA at the A site, forming a new peptide bond. This central reaction of protein synthesis is catalyzed by a peptidyl transferase contained in the large ribosomal subunit. • In step 3, the large subunit moves relative to the mRNA held by the small subunit, thereby shifting the acceptor stems of the two tRNAs to the E and P sites of the large subunit. • In step 4, another series of conformational changes moves the small subunit and its bound mRNA exactly three nucleotides, ejecting the spent tRNA from the E site and resetting the ribosome so it is ready to receive the next aminoacyl-tRNA. • Two elongation factors enter and leave the ribosome during each cycle, each hydrolyzing GTP to GDP and undergoing conformational changes in the process. These factors are called EF-Tu and EF-G in bacteria, and EF1 and EF2 in eukaryotes. • Coupling the GTP hydrolysis-driven changes in the elongation factors to transitions between different states of the ribosome speeds up protein synthesis enormously. • In addition to moving translation forward, EF-Tu increases its accuracy • First, the 16s rRNA in the small subunit of the ribosome assesses the “correctness” of the codon–anticodon match by folding around it and probing its molecular details • When a correct match is found, the rRNA closes tightly around the codon–anticodon pair, causing a conformational change in the ribosome that triggers GTP hydrolysis by EF-Tu. • Incorrect codon–anticodon matches do not readily trigger this conformational change, and these errant tRNAs mostly fall off the ribosome before they can be used in protein synthesis. • There is a short time delay as the amino acid carried by the tRNA moves into position on the ribosome. This time delay is shorter for correct than incorrect codon–anticodon pairs. • An incorrect codon‒ anticodon interaction in the P site of the ribosome (which would occur after the misincorporation) causes an increased rate of misreading in the A site. Successive rounds
- 10. Biology Elite www.biologyelite.com of amino acid misincorporation eventually lead to premature termination of the protein by release factors. The protein made will therefore be degraded and never used. In summary, there are two ways to ensure that right correct amino acid is added: 1. Induced fit: folding of ribosome to ensure the correct geometry 2. Kinetic proofreading: GTP hydrolysis gives weaker interaction between anticodons and tRNA; thus, the delay of getting into position is much longer, causing the misreading and premature ending of translation. Ribosome Structure: Ribosome is made of two thirds of RNA and one third of protein. Ribosomal protein is mainly located in the surface to fill in the gaps and crevices of the folded RNA. Ribosomal protein is mainly used to stabilised the RNA core, while allowing the change in rRNA conformation. It also aids in the initial assembly of the rRNAs that make up the core of the ribosome. Ribosome doesn’t contain easily ionisable function group that can be used to catalyse chemical reaction nor metal ions. We find that 23s rRNA forms a highly structured pocket that, through a network of hydrogen bonds, precisely orient the two reactants and thereby greatly accelerates their covalent joining. Initiation of translation: Eukaryotes: • AUG is the starting codons of translation • Initiator tRNA-methionine complex (Met-tRNAi) is first loaded to small ribosomal unit along with the protein called eukaryotic ignition factors (eIFs). • small ribosomal unit binds to the 5’ end cap of the mRNA, which is previous recognised by eIF4E and eIF4G • small ribosomal unit then move forward to search for AUG • Translation begins at the first encounter AUG. Initiation complex dissociate, allowing large ribosomal unit to assemble and complete the ribosome. • Initiator tRNA remains at the P site, leaving A site empty. • Leaky scanning: small ribosomal unit may skip the first encounter AUG due to subtle difference from its recognition sequence and jump to second AUG. It allows cell to produce closely related protein from one mRNA. Prokaryotes: • Prokaryotic mRNA has no 5’ cap. So the starting direction of translation can not be found. • Bacterial mRNA contains a specific ribosome-binding sequence called Shine-Dalgarno sequence, which located few nucleotide upstream of AUG. • This nucleotide sequence forms a base
- 11. Biology Elite www.biologyelite.com pair with 16s rRNA small ribosomal unit to position the initialing AUG codon into the ribosome. • There are several ribosome binding site in prokaryotic mRNA. So material mRNA is polycistronic: they encode several protein in one mRNA molecule. Termination of translation: • Stop codons signal the end of translation (UAA,UGA,UAG) • Proteins known as release factors bind to any ribosome with a stop codon positioned in the A site, forcing the peptidyl- transferase in the ribosome to catalyze the addition of a water molecule instead of an amino acid to the peptidyl-tRNA • This reaction frees the carboxyl end of the growing polypeptide chain from its attachment to a tRNA molecule, thereby releasing the polypeptide chain • Then the ribosome de-assemble into small and large subunits. Polyribosomes: • it is usual for multiple initiations to take place on each mRNA molecule being translated. As soon as the preceding ribosome has translated enough of the nucleotide sequence to move out of the way, the 5ʹ end of the mRNA is threaded into a new ribosome. • These multiple initiations allow the cell to make many more protein molecules in a given time than would be possible if each protein had to be completed before the next could start. • Because bacterial mRNA does not need to be processed and is accessible to ribosomes while it is being made, ribosomes can attach to the free end of a bacterial mRNA molecule and start translating it even before the transcription of that RNA is complete, following closely behind the RNA polymerase as it moves along DNA. Quality control to prevent translating damaged mRNA: • To avoid translating broken mRNAs, for example, the 5ʹ cap and the poly-A tail are both recognized by the translation-initiation machinery before translation begins nonsense-mediated mRNA decay mechanism:
- 12. Biology Elite www.biologyelite.com • As its 5ʹ end emerges from a nuclear pore, the mRNA is met by a ribosome, which begins to translate it. As translation proceeds, the exon junction complexes (EJCs) that are bound to the mRNA at each splice site are displaced by the moving ribosome. The normal stop codon will lie within the last exon, so by the time the ribosome reaches it and stalls, no more EJCs will be bound to the mRNA. In this case, the mRNA “passes inspection” and is released to the cytosol where it can be translated. • However, if the ribosome reaches a stop codon earlier, when EJCs remain bound, the mRNA molecule is rapidly degraded. In this way, the first round of translation allows the cell to test the fitness of each mRNA molecule as it exits the nucleus. Folding of protein: • Most proteins probably do not fold correctly during their synthesis and require a special class of proteins called molecular chaperones to do so. • Molecular chaperones are useful for cells because there are many different folding paths available to an unfolded or partially folded protein. • Molecular chaperones specifically recognize incorrect, off-pathway configurations by their exposure of hydrophobic surfaces, which in correctly folded proteins are typically buried in the interior. • Chaperones prevent this from happening in normal proteins by binding to the exposed hydrophobic surfaces using hydrophobic surfaces of their own. • Many molecular chaperones are called heat-shock proteins (designated hsp), because they are synthesized in dramatically increased amounts after a brief exposure of cells to an elevated temperature . (42 degrees in 37 degrees of living cells) This reflects the operation of a feedback system that responds to an increase in misfolded proteins. • hsp70: acts early in the life of many proteins (often before the protein leaves the ribosome), with each monomer of hsp70 binding to a string of about four or five hydrophobic amino acids. On binding ATP, hsp70 releases the protein into solution allowing it a chance to re-fold.
- 13. Biology Elite www.biologyelite.com • hsp60: form a large barrel-shaped structure that acts after a protein has been fully synthesized. • To enter a chamber, a substrate protein is first captured via the hydrophobic entrance to the chamber. The protein is then released into the interior of the chamber, which is lined with hydrophilic surfaces, and the chamber is sealed with a lid, a step requiring ATP. Here, the substrate is allowed to fold into its final conformation in isolation, where there are no other proteins with which to aggregate. When ATP is hydrolyzed, the lid pops open , and the substrate protein, whether folded or not, is released from the chamber. • The apparatus that deliberately destroys aberrant proteins is the proteasome, an abundant ATP- dependent protease that constitutes nearly 1% of cell protein. • Each proteasome consists of a central hollow cylinder (the 20S core proteasome) formed from multiple protein subunits that assemble as a stack of four heptameric rings • Each end of the cylinder is normally associated with a large protein complex (the 19S cap) that contains a six-subunit protein ring through which target proteins are threaded into the proteasome core, where they are degraded. • The threading reaction, driven by ATP hydrolysis, unfolds the target proteins as they move through the cap, exposing them to the proteases lining the proteasome core • The proteins that make up the ring structure in the proteasome cap belong to a large class of protein “unfoldases” known as AAA proteins. • It must be able to distinguish abnormal proteins from those that are properly folded. The 19S cap of the proteasome acts as a gate at the entrance to the inner proteolytic core, and only those proteins marked for destruction are threaded through the cap. • A special set of E3 molecules is responsible for the ubiquitylation of denatured or otherwise misfolded proteins, as well as proteins containing oxidized or other abnormal amino acids. Abnormal proteins tend to display on their surface hydrophobic amino acid sequences or conformational motifs that are recognized as degradation signals by these E3 molecules; these sequences are buried and therefore inaccessible in the normal, properly folded version.