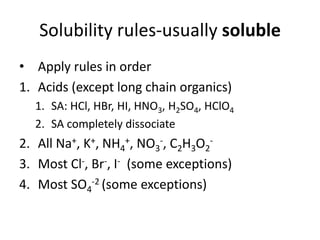
Solubility rules usually soluble
- 1. Solubility rules-usually soluble Apply rules in order Acids (except long chain organics) SA: HCl, HBr, HI, HNO3, H2SO4, HClO4 SA completely dissociate All Na+, K+, NH4+, NO3-, C2H3O2- Most Cl-, Br-, I- (some exceptions) Most SO4-2 (some exceptions)
- 2. Solublity rules –mostly insoluble 5. Most S- 6. Most OH- CO3-2 SO3-2 PO4-3 Strong Bases: Li, Na, K, Rb, Cs OH Ca, Sr, Ba, Ra (OH)2 strong bases completely dissociate 7. Gases Elemental gases very slightly soluble CO2 H2S SO2 slightly soluble (bubble out of solution) H2SO3 and H2CO3 break down in acidic soln See more detailed rule Handouts Book chart
- 3. Another way to look at the rules follows These are only for the most common compounds – there are obviously more rules (and exceptions!!!)
- 4. General Solubility rulesusually soluble Soluble Compounds Salts of Na+ K+ NH4+ Salts of Cl- Br- I- Salt of F- Salts of NO3- ClO3- ClO4- and C2H3O2- Salts of SO4-2 Exceptions Ag+ Hg2+2 Pb+2 Mg+2 Ca+2 Sr+2 Ba+2 Pb+2 Sr+2 Ba+2 Pb+2
- 5. General solubility rulesgenerally insoluble Insoluble compounds Salts of CO3-2 PO4-3 C2O4-2 (oxalate) CrO4-2 S-2 OH-1 O-2 Exceptions Salts of NH4+ and all the alkali metals (Group IA metals)
- 6. Medical applications Kidney stones are often calcium salts of slightly soluble compounds (we classified them as insoluble) Phosphates Carbonates Oxalates Calcium oxalate crystals are needle like and would be extremely painful to excrete!! Whereas the phosphates and carbonates aren’t needle-like
- 7. Other medical applications A lot of drugs have poor solubilities as neutral molecules – Many of the drug molecules have acidic or basic groups If you react the acid molecules with NaOH – you can produce the sodium salt - usually with better solubility If you react the basic molecules (the amines) with HCl – you produce the chloride salt and usually increase the solubility
- 8. Example of these drugs Naproxen sodium (or sodium naproxen) the drug in Alleve – acid group reacts with NaOH to eliminate water leaving naproxen as an anion Naproxen-COOH + NaOH NaproxenCOO-Na+ + H2O Pseudoephedrine hydrochloride:a decongestant PseudoephedrineNH2 + HCl pseudoephedreinNH3+Cl-