Ceramics materials prop thermal and mechanical
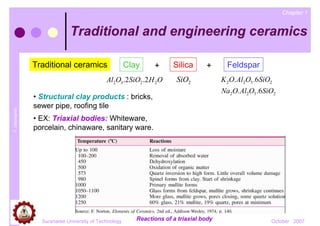
- 1. Chapter 1 Traditional and engineering ceramics Traditional ceramics Clay + Silica + Feldspar Al2O3 .2 SiO2 .2 H 2O SiO2 K 2O. Al2O3 .6 SiO2 Na2O. Al2O3 .6SiO2 • Structural clay products : bricks, sewer pipe, roofing tile T. Udomphol • EX: Triaxial bodies: Whiteware, porcelain, chinaware, sanitary ware. Suranaree University of Technology Reactions of a triaxial body October 2007
- 2. Chapter 1 Traditional and engineering ceramics Traditional ceramics Triaxial whiteware chemical composition T. Udomphol Suranaree University of Technology October 2007
- 3. Chapter 1 Traditional and engineering ceramics Traditional ceramics T. Udomphol Suranaree University of Technology October 2007
- 4. Chapter 1 Traditional and engineering ceramics Traditional ceramics quartz Mullite needles T. Udomphol High silica glass Electron micrograph of an electrical insulator porcelain (etched 10 s, 0oC, 40% HF, silica replica) Suranaree University of Technology October 2007
- 5. Chapter 1 Traditional and engineering ceramics Master and plaster moulds Slip casting process Pottery T. Udomphol Slip casting Fire Colour paint Dry http://www.lindawilsonceramics.co.za/3.html Suranaree University of Technology Fresh cast October 2007
- 6. Chapter 1 Traditional and engineering ceramics Slip casting process Sanitaryware Hemihydrate plaster – produced from gymsum 150o C CaSO4 .2 H 2O → CaSO4 . 1 H 2O + 3 H 2O 2 2 Slip preparation T. Udomphol in ball mill www.3emmegi.com Suranaree University of Technology Slip casting in plaster moulds and demoulding October 2007
- 7. Chapter 1 Traditional and engineering ceramics Engineering ceramics • Contain more of pure compounds of oxides, carbides, nitrides. • Ex: Al2O3, Si3N4, SiC, ZrO2 , refractory oxides T. Udomphol Mechanical properties of engineering ceramics Suranaree University of Technology October 2007
- 8. Chapter 1 Traditional and engineering ceramics Engineering ceramics Alumina • Refractory tubing • High purity crucibles for high temp • High quality electrical applications T. Udomphol (low dielectric loss and high resistivity) • Spark plug insulator www.sentrotech.com Microstructure of sintered, powdered aluminium oxide doped with magnesium oxide Alumina tubes Suranaree University of Technology October 2007
- 9. Chapter 1 Traditional and engineering ceramics Engineering ceramics Silicon nitride (Si3N4) • Dissociate at T > 1800oC. N2 flow • Cannot be directly sintered reaction bonding. nitriding T. Udomphol Silicon powder Microporous Si3N4 Hot pressing with 1-5%MgO High strength nonporous Si3N4 www.defazio-rotary.com Suranaree University of Technology Silicon nitride for engineering applications October 2007
- 10. Chapter 1 Traditional and engineering ceramics Engineering ceramics Silicon carbide (SiC) • Hard refractory carbide. www.stork.com • Form skin of SiO2 at high temp. T. Udomphol • Resistance to oxidation at high temp. • Can be sintered 2100oC with 0.5-1%B. • Fibrous reinforcement in ceramic- matrix composite material. SiC fibre reinforced Titanium matrix Suranaree University of Technology October 2007
- 11. Chapter 1 Traditional and engineering ceramics www.azom.com Engineering ceramics Zirconia (ZrO2) 1170oC T. Udomphol • Polymorphic: tetragonal monoclinic. Volume expansion Zirconia Heat treatment Cubic structure • Mixed with CaO, MgO and Y2O3 Partially stabilized zirconia (PSZ). Suranaree University of Technology October 2007
- 12. Chapter 1 Mechanical properties of ceramics • Brittle • High strength (varying from 0.7 – 7000 MPa) • Better compressive strength than tensile (5-10 times) Level of strength Materials (MPa) T. Udomphol > 1000 polycrystalline long ceramic fibres (Al2O3 , SiC): 1-2 GPa, single crystal short ceramic fibres (Al2O3 , SiC whiskers): 5-20 GPa, 600-1000 Hot Pressed structural ceramics such as silicon nitride, silicon carbide, alumina; sintered tetragonal zirconia and sialon; cemented carbides 200-600 sintered pure alumina and SiC; tempered glass 100-200 impure and/or porous alumina; mullite; high-alumina porcelains; reaction bonded silicon nitride and carbide; glass ceramics 50-100 porcelains; steatite, cordierite; magnesia, polished glasses; <50 refractory; porous ceramics; glasses Suranaree University of Technology October 2007
- 13. Chapter 1 Mechanical properties of ceramics Deformation mechanisms • Lack of plasticity due to ionic and covalent bonding (directional). • Stressing of covalent crystal separation of electron-pair bonds without subsequent reformation brittle T. Udomphol • Deforming of ionic single crystal (MgO or NaCl) shows considering amount of plastic deformation under compressive force. However ionic polycrystals are brittle due to crack formation at grain boundaries. NaCl structure showing slip on the (110) plane [110] direction or AA’ and on the (100) plane [010] direction BB’ Suranaree University of Technology October 2007
- 14. Chapter 1 Mechanical properties of ceramics Factors affecting strength of ceramics Should control • chemical composition Depending on amount of defects • microstructure giving stress concentration • surface condition • temperature • environment T. Udomphol • Surface cracks • Porosity Fabrication • Inclusions • Excessive grain sizes Note: No plastic deformation during crack propagation from defects very brittle. Suranaree University of Technology October 2007
- 15. Chapter 1 Mechanical properties of ceramics Toughness of ceramics • Low toughness due to covalent-ionic bonding. • Using hot pressing, reaction bonding to improve toughness. • Fibre-reinforced ceramic matrix composites. T. Udomphol Fracture toughness of ceramics Suranaree University of Technology October 2007
- 16. Chapter 1 Mechanical properties of ceramics Toughness of ceramics Example A reaction-bonded silicon nitride has a strength of 300 MPa and a fracture toughness of 3.6 MPa.m1/2, What is the largest-size internal crack that this material can support without fracturing? Given Y = 1 T. Udomphol K IC = Yσ f πa a= K 2 IC = (3.6MPa. m ) 2 πσ 2 f π (300MPa )2 a = 4.58 ×10 −5 m = 45.8µm Therefore the largest internal crack 2a = 91.6 µm Suranaree University of Technology October 2007
- 17. Chapter 1 Mechanical properties of ceramics Transformation toughening of Partially Stabilized Zirconia (PSZ) Sintering at 1800oC+rapid cooling to RT+ Zirconia reheating at 1400oC to give fine precipitates + (CaO, MgO or Y2O3) PSZ (metal stable) T. Udomphol Volume expansion Tetragonal monoclinic under stressing Suranaree University of Technology October 2007
- 18. Chapter 1 Mechanical properties of ceramics Fatigue failure of ceramics • Fatigue failure in ceramics is rare due to lack of plastic deformation during cyclic loading. T. Udomphol Fatigue cracking of polycrystalline alumina under cyclic loading Suranaree University of Technology October 2007
- 19. Chapter 1 Mechanical properties of ceramics Abrasive property of ceramics • Hard and brittle • Used as cutting, grinding and polishing tools. T. Udomphol • Aluminium oxide • Silicon carbide • Titanium nitride • Tungsten carbide • Boron nitride www.moldmakingtechnology.com Ceramic cutting tools Ceramic grinding wheels Suranaree University of Technology October 2007
- 20. Chapter 1 Thermal properties of ceramics • Low thermal conductivity due to ionic-covalent bonding insulator. T. Udomphol • Also used as refractories in metal, chemical and glass industries. Thermal conductivity of ceramic materials Suranaree University of Technology October 2007
- 21. Chapter 1 Thermal properties of ceramics Ceramic refractory materials img.alibaba.com • A mixture of ceramic compounds • Low-high temperature strength • Low bulk density (2.1-3.3 g.cm-3) T. Udomphol • Porosity insulating Acidic refractory Mainly based on SiO2 and Al2O3 Basic refractory Refractory bricks (60% Al2O3) Mainly based on magnesia (MgO), for hot blast furnace lime (CaO) and Cr2O3 Suranaree University of Technology October 2007
- 22. Chapter 1 Thermal properties of ceramics T. Udomphol Suranaree University of Technology October 2007
- 23. Chapter 1 Thermal properties of ceramics Acidic refractory Basic refractory • Silica refractory has high • Basic refractory consists of refractoriness, high mechanical mixtures of MgO, CaO and Cr2O3. strength and rigidity at high • High bulk density T. Udomphol temperature. • High melting point • Fireclays (fine plastic clays + • Good resistance to chemical flint + coarse clay or grog) attack (basic slag, oxides) • High alumina refractories • Ex 92-95% MgO used for lining contains 50-99% alumina, in basic-oxygen steelmaking giving higher fusion temperature process (more expensive than fireclay). Suranaree University of Technology October 2007
- 24. Chapter 1 Thermal properties of ceramics Ceramic tile insulation for the space shuttle orbiter • About 24,000 ceramic tiles (70%) of silica-fibre compound are used for insulating external surface of space shuttle. T. Udomphol Suranaree University of Technology October 2007
- 25. Chapter 1 Thermal properties of ceramics media.nasaexplores.com Ceramic tile insulation for the space shuttle orbiter • High temperature reusable surface (HTRS) made from 90% silica fibres and 10% empty space. T. Udomphol • Density = 0.144 g.cm-3 • Temp ~ 1260oC Borosilicate coating Microstructure of LI900 high-temperature upload.wikimedia.org reusable surface insulation (HTRS) Suranaree University of Technology October 2007
- 26. Chapter 1 Glass www.geocities.com Definition of glass • An inorganic and noncrystalline material which maintains its amorphous microstructure below its glass transition temperature. T. Udomphol Blown glass Properties of glass www.arch.tu.ac.th • Transparency • Hardness and strength • Corrosion/chemical resistance • Vacuumtight enclosure • Insulator Tinted or heat-absorbed glass Suranaree University of Technology October 2007
- 27. Chapter 1 Glass Glass transition temperature (Tg) • Unlike solidified metal, a glass liquid does not crystallize but follow an AD path. T. Udomphol Temp (decrease) Viscous Plastic Glassy • The faster cooling rate, the higher values of Tg. Solidification of crystalline and amorphous materials showing a change in specific volume Suranaree University of Technology October 2007
- 28. Chapter 1 Glass Structure of glass Glass forming oxide - SiO2 T. Udomphol Si-O tetrahedron Ideal crystalline silica Simple silica glass with (crystobalite) no-long range order Suranaree University of Technology October 2007
- 29. Chapter 1 Glass Structure of glass Glass modifying oxides - Na2O, K2O, CaO, MgO • Oxygen from Na2O breaks up T. Udomphol silica network, leaving oxygen atoms with an unshared electron. • Na+ or K+ ions fits into interstices of network. Network modified glass (soda-lime glass) Suranaree University of Technology October 2007
- 30. Chapter 1 Glass Structure of glass Intermediate oxides in glass - Al2O3 , Pb2O3 • Oxides such as Al2O3 or Pb2O3 cannot form glass network but T. Udomphol join into an existing network. • Aluminosilicate glass provides higher temperature than common glass. Suranaree University of Technology October 2007
- 31. Chapter 1 Glass Glass composition • Silica glass No radiation damage • Soda-lime glass T. Udomphol Reduced Tm ~ 730 oC • Borosilicate glass (Pyrex glass) Low thermal expansion • Lead glass Shielding from high energy radiation Suranaree University of Technology October 2007
- 32. Chapter 1 Glass Viscous deformation of glasses • Glass remains its viscous (supercooled) liquid above Tg. T. Udomphol Temp > Tg Viscosity η = ηo e + Q RT η = viscosity of the glass ηo = pre-exponential constant Q = molar activation energy for viscous flow R = gas constant T = absolute temperature Suranaree University of Technology October 2007
- 33. Chapter 1 Glass Viscosity reference points Working point Viscosity = 104 poise (103 Pa.s) fabrication T. Udomphol Softening point Viscosity = 108 poise glass flows at an appreciate rate under its own weight (and surface tension). Annealing point Viscosity = 1013 poise relieving internal stresses Viscosity = 1014.5 poise glass is rigid with slow Strain point rate of stress relaxation. Note: glass are usually melt at temp relating to viscosity = 102 poise Suranaree University of Technology October 2007
- 34. Chapter 1 Glass Example A 96 % silica glass has a viscosity of 1013 P at its annealing point of 940oC and a viscosity of 108 P at its softening point of 1470oC. Calculate the activation energy in kJ/mol for the viscous flow of this glass in this temperature range. Tanneal = 940+273 = 1213 K, ηap =1013 P η = η o e + Q RT T. Udomphol Tsoftening = 1470+273 = 1743 K, ηap =108 P η ap Q 1 1 1013 = exp − = = 105 η sp R Tap Tsp 108 Q 1 1 10 = exp 5 − 8.314 1213K 1743K Q = 382kJ / mol Suranaree University of Technology October 2007
- 35. Chapter 1 Glass Fabrications of glass • Forming sheet and plate glass • Float glass process molten glass ribbon moves on the top of molten tin in a reducing atmosphere. T. Udomphol • Remove glass sheet when the glass surface is hard enough then pass to annealing furnace called lehr to remove residual stresses. • Blowing, pressing and casting of glass • For deep, hallow shapes like bottles, jars, light bulbs envelops. • Blowing air to force molten glass into moulds. • Pressing a plunger into a mold containing molten glass. • Casting into open moulds. Suranaree University of Technology October 2007
- 36. Chapter 1 Glass T. Udomphol Float glass process Suranaree University of Technology October 2007
- 37. Chapter 1 Glass T. Udomphol a) Reheat , b) final blow stage of a glass blowing machine process Suranaree University of Technology October 2007
- 38. Chapter 1 Glass Pyrex glass • Borosilicate glass • Low thermal expansion • Inert to almost all materials with the exception of hydrofluoric acid, hot phosphoric acid and hot alkalies. T. Udomphol Approximate composition SiO2 81% Na2O 4.0% K2O 0.5 B2O3 13.0% Al2O3 2.0% Suranaree University of Technology October 2007
- 39. Chapter 1 Glass Tempered glass The surface cools first (by rapid air cooling) and contract while the interior is warm, developing compressive on the surface and tensile in the middle. T. Udomphol a) After surface has cooled from high b) after centre has cooled. temperature near glass-softening temperature. Suranaree University of Technology October 2007
- 40. Chapter 1 Glass Tempered glass • Tempering effect increases the strength (4 x stronger than annealed glass. T. Udomphol • Has higher impact resistance than annealed glass. • Ex: Auto side window, safety glass for doors. Distribution of residual stresses across the sections of glass thermally tempered and chemically strengthend Suranaree University of Technology October 2007
- 41. Chapter 1 Glass Laminated glass www.dupont.com • Plastic interlayer (PVB-poly vinyle butyral) is sandwiched with floated/annealed glass. • Safety glass: Breaking like a spider web. T. Udomphol http://en.wikipedia.org/ Laminated glass Spider web breaking pattern Suranaree University of Technology October 2007
- 42. Chapter 1 Glass Laminated glass T. Udomphol www.goodandquickglass.com Suranaree University of Technology October 2007
- 43. Chapter 1 Glass Used in supersonic aircraft glazing, Chemical strengthened glass ophthalmic lenses. • Submerging sodium aluminosilicate glass in a bath containing a potassium salt at T~ 450-500oC for 6-10 h. • Replacing Na ions with T. Udomphol larger K ions on the glass surface. Producing thin compressive stresses at the surface and tensile stresses in the centre. Distribution of residual stresses across the section of glass Suranaree University of Technology thermally tempered and chemically strengthened. October 2007