Ecology b-cycle
- 2. Objectives: • Identify the flow on each biogeochemical cycle. • Explain the impact that humans have on the biogeochemical cycles.
- 3. Biogeochemical cycles • Biogeochemical cycles: The chemical interactions (cycles) that exist between the atmosphere, hydrosphere, lithosphere, and biosphere. • Biogeochemical cycles are components of the broader cycle that govern the functioning of planet Earth
- 5. Biogeochemical cycles • The transfer of matter involves biological, geological and chemical processes; hence the name biogeochemical cycles derives. Biogeochemical cycles may also be referred to as cycles of nature because they link together all organisms and abiotic features on earth (see Figure at next slide). Matter is continually recycled among living and abiotic elements on earth.
- 7. • biogeochemical cycles facilitate the transfer of matter from one form to another and from one location to another on planet earth. Additionally, biogeochemical cycles are sometimes called nutrient cycles, because they involve the transfer of compounds that provide nutritional support to living organisms.
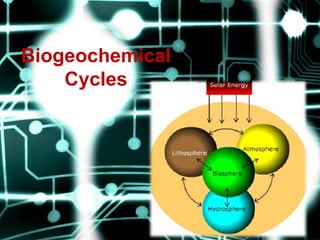
- 8. PathwaysofBiogeochemicalCycles • Parts that comprise planet earth have been categorized into four spheres (regions). One is the sphere which has life and it is called the biosphere (it is the region occupied by living organisms such as plants, animals, fungi) and the other three spheres are largely devoid of life, they include;
- 9. • lithosphere (region occupied by soil, land and the earth crust), atmosphere (air and space) and hydrosphere (areas covered by water such as rivers, lakes and oceans). However, where the biosphere overlaps the lithosphere, atmosphere or hydrosphere, there is a zone occupied by living organisms.
- 10. Categories of biogeochemical cycles • Biogeochemical cycles differ in their pathways, and on this basis the biogeochemical cycles have been categorized into two: Sedimentary cycles: Phosphorus cycle Sulfur cycle Gaseous cycles Carbon cycle Oxygen cycle Nitrogen cycle Hydrological cycle
- 11. Sedimentary cycles: • these cycles involve the transportation of matter through the ground to water; that is to say from the lithosphere to the hydrosphere.
- 13. Phosphorus cycle Phosphorus is commonly found in water, soil and sediments. Phosphorus cannot be found in air in the gaseous state. This is because phosphorus is usually a liquid at standard temperatures and pressures. Phosphorus is mainly cycled trough water, soil and sediments. However, very small particles in the atmosphere may contain phosphorus or its compounds. Phosphorus moves slowly from deposits on land and in sediments, to living organism , and much more slowly back into the soil and water sediment. The phosphorus cycle is the slowest one of the
- 14. Fig. 3-31, p. 77 Dissolved in Ocean Water Marine Sediments Rocks uplifting over geologic time settling out weatheringsedimentation Land Food Webs Dissolved in Soil Water, Lakes, Rivers death, decomposition uptake by autotrophs agriculture leaching, runoff uptake by autotrophs excretion death, decomposition mining Fertilizer weathering Guano Marine Food Webs
- 15. The cycle basically starts out in the earth’s soil. The soil contains phosphate and when something grows out of the soil it should have phosphate as well. When the plants grow they are consumed by herbivore and omnivore animals The animal’s waste or the animal’s body when it dies becomes detritus. Detritus is non- living organic material. When the detritus goes deep into the soil, detritivores in the soil decompose and become the soil’s phosphate and the cycle repeats.
- 16. Sulfur cycle • Sulfur in its natural form is a solid, and restricted to the sedimentary cycle in this form. It is transported by physical processes like wind, erosion by water, and geological events like volcanic eruptions. However, in its compounds such as sulfur dioxide, sulfuric acid, salts of sulfate or organic sulfur, sulfur can be moved from the ocean to the atmosphere, to land and then to the ocean through rainfall and rivers.
- 17. Fig. 3-32, p. 78 Hydrogen sulfide Sulfur Sulfate salts Decaying matter Animals Plants Ocean Industries Volcano Hydrogen sulfide Oxygen Dimethyl sulfide Ammonium sulfate Ammonia Acidic fog and precipitationSulfuric acid WaterSulfur trioxide Sulfur dioxide Metallic sulfide deposits
- 18. Effects of Human Activities on the Sulfur Cycle • We add sulfur dioxide to the atmosphere by: –Burning coal and oil –Refining sulfur containing petroleum. –Convert sulfur-containing metallic ores into free metals such as copper, lead, and zinc releasing sulfur dioxide into the environment.
- 19. Gaseous cycles • these involve the transportation of matter through the atmosphere. Common example of gaseous cycles are:
- 21. Carbon cycle • Carbon is one of the most important elements that sustain life on earth. Carbon dioxide and methane gases (compounds of carbon) in the earth's atmosphere has a substantial effect on earth's heat balance. It absorbs infrared radiation and hence may contribute to global warming and climate change.
- 22. MARINE CARBON CYCLE Slide 35Slide 35Slide 35 Diffusion between atmosphere and ocean Carbon dioxide dissolved in ocean water Marine food webs Producers, consumers, decomposers, detritivores Marine sediments, including formations with fossil fuels Combustion of fossil fuels incorporation into sediments death, sedimentation uplifting over geologic time sedimentation photosynthesis aerobic respiration Figure 4-29a Page 78
- 23. photosynthesis aerobic respirationTerrestrial rocks Soil water (dissolved carbon) Land food webs producers, consumers, decomposers, detritivores Atmosphere (most carbon is in carbon dioxide) Peat, fossil fuels combustion of wood (for clearing land; or for fuel sedimentation volcanic action death, burial, compaction over geologic timeleaching runoff weathering Combustion of fossil fuels TERRESTRIAL CARBON CYCLE
- 24. Carbon Cycle Diagram Carbon in Atmosphere Plants use carbon to make food Animals eat plants and take in carbon Plants and animals die Decomposers break down dead things, releasing carbon to atmosphere and soil Bodies not decomposed — after many years, become part of oil or coal deposits Fossil fuels are burned; carbon is returned to atmosphere Carbon slowly released from these substances returns to atmosphere
- 25. The Carbon Cycle
- 26. • Fossil fuels release carbon stores very slowly • Burning anything releases more carbon into atmosphere — especially fossil fuels • Increased carbon dioxide in atmosphere increases global warming • Fewer plants mean less CO2 removed from atmosphere Human Impact
- 27. Nitrogen cycle • Nitrogen gas is the most abundant element in the atmosphere and all the nitrogen found in terrestrial ecosystems originate from the atmosphere. The nitrogen cycle is by far the most important nutrient cycle for plant life.
- 30. Effects of Human Activities on the Nitrogen Cycle • We alter the nitrogen cycle by: –Adding gases that contribute to acid rain. –Adding nitrous oxide to the atmosphere through farming practices which can warm the atmosphere and deplete ozone. –Contaminating ground water from nitrate ions in inorganic fertilizers. –Releasing nitrogen into the troposphere through deforestation.
- 31. Effects of Human Activities on the Nitrogen Cycle • Human activities such as production of fertilizers now fix more nitrogen than all natural sources combined. Figure 3-30Figure 3-30
- 32. Oxygen cycleOxygen cycle • The oxygen cycle describes the movement of oxygen within and between its three main reservoirs: the atmosphere, the biosphere, and the lithosphere. The main driving factor of the oxygen cycle is photosynthesis and because of this, oxygen and carbon cycles are usually linked and the two cycles are collectively called oxygen-carbon cycle.
- 33. Fig. 3-26, p. 72 PrecipitationPrecipitation Transpiration Condensation Evaporation Ocean storage Transpiration from plants Precipitation to land Groundwater movement (slow) Evaporation from land Evaporation from ocean Precipitation to ocean Infiltration and Percolation Rain clouds Runoff Surface runoff (rapid) Surface runoff (rapid) Photosynthesis
- 34. respiration Rabbit eats food, breaks it down and releases CO2. Plant uses CO2 to make food. Rabbit gives off CO2, which is taken in by the plant. Plant gives off O2, which is taken in by the rabbit.
- 35. How are photosynthesis and cellular respiration similar? • • Photosynthesis uses carbon dioxide and produces oxygen. • Cellular respiration uses oxygen and produces carbon dioxide.
- 36. All Animals and Other Consumers Use Oxygen • We use oxygen to break down simple sugar and release energy. • This can be done through respiration or fermentation. • Animals mainly use respiration.
- 38. Hydrological cycle • This is some times called the water cycle. Water is the most important chemical of life for all living organisms on earth. Water in the atmosphere is usually in form of vapor but condenses to liquid water and can solidify when temperatures are 00 C to form ice. Ninety three percent of water on earth is in solid state mainly comprising the ice caps and glaciers of Polar Regions.
- 40. • The earth has a limited amount of water. That water keeps going around and around and around and around and (well, you get the idea) in what we call the "Water Cycle". This cycle is made up of a few main parts: • Evaporation or (Transpiration) • Condensation • Precipitation • Accumulation or (Collection) • Ground Water • Saturation • Infiltration
- 41. • Evaporation is when the sun heats up water in rivers, lakes or the ocean and turns it into vapor or steam. The water vapor or steam leaves the river, lake or ocean and goes into the air. Evaporation
- 42. • Water vapor in the air gets cold and changes back into liquid, forming clouds. This is called condensation. • You can see this at home when you take a shower and the windows and mirrors in the bathroom fog up. You can also do this by breathing on a mirror. Condensation
- 43. • Precipitation occurs when so much water has condensed that the air cannot hold it anymore. The clouds get heavy and water falls back to the earth in the form of rain, hail, sleet or snow. Precipitation
- 44. • When water falls back to earth as precipitation, it may fall back in the oceans, lakes or rivers or it may end up on land. When it ends up on land, it will either soak into the earth (infiltration) and becomes part of the “ground water” that plants and animals use to drink or it may run over the soil and collect in the oceans, lakes or rivers where the cycle starts all over again. Accumulation
- 45. HUMAN IMPACTS TO WATER CYCLE 1. Water withdrawal from streams, lakes and groundwater. (salt water intrusion and groundwater depletion) 2. Clear vegetation from land for agriculture, mining, road and building construction. (nonpoint source runoff carrying pollutants and reduced recharge of groundwater) 3. Degrade water quality by adding nutrients(NO2, NO3, PO4) and destroying wetlands (natural filters). 4. Degrade water clarity by clearing vegetation and
- 46. Nature of elements transported in biogeochemical cycles • When living organisms die and decay, their body structures disintegrate and may be reduced to constituent molecules. Depending on the region where disintegration of the organisms occurred, the component molecular elements then join the biogeochemical cycle.
- 47. Elements transported in the biogeochemical cycles have been categorized as: • 1. Micro elements – these are elements required by living organisms in smaller amounts. Examples of such elements include boron used mainly by green plants, copper used by some enzymes and molybdenum used by nitrogen-fixing bacteria. • 2. Macro elements – these are elements required by living organisms in larger amounts. Examples of such elements include carbon, hydrogen, oxygen, nitrogen, phosphorous, sulfur.
- 48. The importance of biogeochemical cycles • Biogeochemical cycles serve a variety of functions at ecosystem level and in ensuring survival of various organisms including humans. Below are some of the importance's of biogeochemical cycles.
- 49. Importance'sofBiogeochemicalCycles • enable the transformation of matter from one form to another. • enable the transfer of molecules from one locality to another. • facilitate the storage of elements • assists in functioning of ecosystems. • cycles link living organisms with living organisms, living organisms with the non living organisms and nonliving organisms with non living organism. • regulate the flow of substances.
Notas del editor
- Figure 3.31 Natural capital: simplified model of the phosphorus cycle. Phosphorus reservoirs are shown as boxes; processes that change one form of phosphorus to another are shown in unboxed print. QUESTION: What are three ways in which your lifestyle directly or indirectly affects the phosphorus cycle?
- Figure 3.32 Natural capital: simplified model of the sulfur cycle. The movement of sulfur compounds in living organisms is shown in green, blue in aquatic systems, and orange in the atmosphere. QUESTION: What are three ways in which your lifestyle directly or indirectly affects the sulfur cycle?
- Figure 3.26 Natural capital: simplified model of the hydrologic cycle.
- Biogeochemical cycles enable the transformation of matter from one form to another. This transformation enables the utilization of matter in a form specific to particular organisms. For example humans utilize water in liquid form. Through the hydrological cycle, water vapour is condensed to liquid and ice converted to liquid water. Nitrogen, despite its abundance in the atmosphere it’s often the most limiting nutrient for plant growth. This problem occurs because most plants can only take up nitrogen in two solid forms: ammonium ion (NH4+) and the ion nitrate (NO3-). Therefore, biogeochemical cycles enable the provision of elements to organisms in utilizable forms. Biogeochemical cycles enable the transfer of molecules from one locality to another. Some elements such as nitrogen a re highly concentrated in the atmosphere, but some of the atmospheric nitrogen is transferred to soil through the nitrogen cycle (which is a biogeochemical cycle). Biogeochemical cycles facilitate the storage of elements. Elements carried through the biogeochemical cycles are stored in their natural reservoirs, and are released to organisms in small consumable amounts. For example through the nitrogen cycle and with the help of the nitrogen fixing bacteria, green plants are able to utilize nitrogen in bits though it is abundant in the atmosphere. Biogeochemical cycles assists in functioning of ecosystems. An ecosystem is a system that properly functions in a state of equilibrium, and when ever any imbalances occur, the ecosystem through the biogeochemical cycles restores to the equilibrium state; this may take a few days or many years. The adjustment is such that the disturbing factor is eliminated. Biogeochemical cycles link living organisms with living organisms, living organisms with the non living organisms and nonliving organisms with non living organism. This is because all organisms depend on one another and most especially, the biotic (living component) and a biotic component of the ecosystem are linked by flow on nutrients engineered by the biogeochemical cycles. Biogeochemical cycles regulate the flow of substances. Since the biogeochemical cycles pass through different spheres, the flow of elements is regulated because each sphere has a particular medium and the rate at which elements flow is determined by the viscosity and density of the medium. Therefore elements in the biogeochemical cycles flow at differing rates with in the cycle and this regulates the flow of the elements in those cycles.
