Lecture 4.3- Isotopes
•Descargar como ZIP, PDF•
7 recomendaciones•7,419 vistas
Section 4.3 Lecture for Honors & Prep Chemistry
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
Grade 8 Chemistry Structure of Matter : Atoms, Molecules and Ions

Grade 8 Chemistry Structure of Matter : Atoms, Molecules and Ions
Destacado
Destacado (18)
multiple choice exam in both Biology and Chemistry

multiple choice exam in both Biology and Chemistry
Periodic Table of the Elements Quiz Game, Lesson PowerPoint

Periodic Table of the Elements Quiz Game, Lesson PowerPoint
Chemical Structure: Structure of Matter. Elements, Ions & Isotopes 

Chemical Structure: Structure of Matter. Elements, Ions & Isotopes
Similar a Lecture 4.3- Isotopes
Similar a Lecture 4.3- Isotopes (20)
Ch. 3 elements and the periodic table(sec.1,2and 3)

Ch. 3 elements and the periodic table(sec.1,2and 3)
Periodic table of elements by Muhammad Fahad Ansari 12IEEM14

Periodic table of elements by Muhammad Fahad Ansari 12IEEM14
Más de Mary Beth Smith
Más de Mary Beth Smith (20)
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Proteins (part a)
Último
This presentation was provided by William Mattingly of the Smithsonian Institution, during the third segment of the NISO training series "AI & Prompt Design." Session Three: Beginning Conversations, was held on April 18, 2024.Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"National Information Standards Organization (NISO)
This presentation was provided by William Mattingly of the Smithsonian Institution, during the fourth segment of the NISO training series "AI & Prompt Design." Session Four: Structured Data and Assistants, was held on April 25, 2024.Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"National Information Standards Organization (NISO)
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
Último (20)
Interactive Powerpoint_How to Master effective communication

Interactive Powerpoint_How to Master effective communication
Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"
IGNOU MSCCFT and PGDCFT Exam Question Pattern: MCFT003 Counselling and Family...

IGNOU MSCCFT and PGDCFT Exam Question Pattern: MCFT003 Counselling and Family...
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
Lecture 4.3- Isotopes
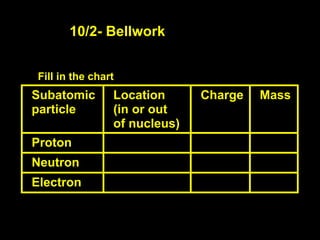
- 1. 10/2- Bellwork Fill in the chart Subatomic Location Charge Mass particle (in or out of nucleus) Proton Neutron Electron
- 2. Elements are different because they contain different numbers of protons. The atomic number of an element is the number of protons in the nucleus.
- 4. For neutral atoms PROTONS = ELECTRONS
- 5. ISOTOPES-Atoms with the same number of protons, but different numbers of neutrons
- 6. ISOTOPES-Atoms with the same number of protons, but different numbers of neutrons They are still the same element, but they have different weights because they have different numbers of neutrons.
- 7. The number of protons plus neutrons in an atom is called the mass number.
- 8. The number of protons plus neutrons in an atom is called the mass number. Isotopes are identified by their mass numbers. Mass number Iodine-131 or 131 53 I atomic number
- 9. Isotopes of an element will have the same _____________ but different ____________.
- 10. Isotopes of an element will have the same _____________ but different ____________. atomic number
- 11. Isotopes of an element will have the same _____________ but different ____________. atomic number mass numbers
- 12. Isotopes are chemically alike because they have identical numbers of protons and electrons.
- 13. Au is the chemical symbol for gold.
- 14. Au is the chemical symbol for gold. protons + neutrons
- 15. Au is the chemical symbol for gold. protons + neutrons protons
- 16. Au is the chemical symbol for gold. protons + neutrons protons
- 17. Au is the chemical symbol for gold. protons + neutrons 118 protons
- 18. Au is the chemical symbol for gold. protons + neutrons 118 protons neutrons
- 19. 4.1
- 20. for Sample Problem 4.1
- 22. for Conceptual Problem 4.2
- 24. The atomic mass of an element is a weighted average, which accounts for how much of each isotope occurs naturally.
- 25. The atomic mass of an element is a weighted average, which accounts for how much of each isotope occurs naturally.
- 26. 4.3 Some Elements and Their Isotopes
- 30. for Conceptual Problem 4.3 for Conceptual Problem 4.3
- 31. The periodic table arranges elements into groups based on a set of repeating properties.
- 32. •Each horizontal row of the periodic table is called a period. • Within a given period, the properties of the elements vary as you move across it from element to element. • At the end of a sentence, written horizontally, is a period.
- 33. 4.3 The Periodic Table—A Preview A Period
- 34. 4.3 The Periodic Table—A Preview A Period
- 36. •Each vertical column of the periodic table is called a group, or family.
- 37. •Each vertical column of the periodic table is called a group, or family. •Elements within a group have similar chemical and physical properties.
- 38. 4.3 The Periodic Table—A Preview A Group or Family
- 39. 4.3 The Periodic Table—A Preview A Group or Family
- 40. 4.3 Section Quiz 1. Isotopes of an element have a. the same mass number. b. different atomic numbers. c. the same number of protons but different numbers of neutrons. d. the same number of protons but different numbers of electrons.
- 41. 4.3 Section Quiz 1. Isotopes of an element have a. the same mass number. b. different atomic numbers. c. the same number of protons but different numbers of neutrons. d. the same number of protons but different numbers of electrons.
- 42. 4.3 Section Quiz 2. How many neutrons are in sulfur-33? a. 16 neutrons b. 33 neutrons c. 17 neutrons d. 32.06 neutrons
- 43. 4.3 Section Quiz 2. How many neutrons are in sulfur-33? a. 16 neutrons b. 33 neutrons c. 17 neutrons d. 32.06 neutrons
Notas del editor
- Neon-20, neon-21, and neon-22 are three isotopes of neon, a gaseous element used in lighted signs. Comparing and Contrasting How are these isotopes different? How are they similar?
- Au is the chemical symbol for gold. Applying Concepts How many electrons does a gold atom have?
- ANS:CPTS:1REF:p. 112OBJ:4.3.1
- ANS:CPTS:1REF:p. 111OBJ:4.3.2