Electrochemistry 1
•Descargar como PPT, PDF•
0 recomendaciones•312 vistas
this is very useful for engineering student
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
9.substituent, effect of substituents on reactivity& orientation .

9.substituent, effect of substituents on reactivity& orientation .
Nucleophilic substitution sn1 sn2 nucleophile halogenoalkane in organic chemi...

Nucleophilic substitution sn1 sn2 nucleophile halogenoalkane in organic chemi...
photo chemistry of ligand in coordination compound

photo chemistry of ligand in coordination compound
Similar a Electrochemistry 1
Similar a Electrochemistry 1 (20)
Electrochemistry Introduction electrochemical cells

Electrochemistry Introduction electrochemical cells
New chm-152-unit-8-power-points-sp13-140227172047-phpapp01

New chm-152-unit-8-power-points-sp13-140227172047-phpapp01
3rd Lecture on Electrochemistry | Chemistry Part I | 12th Std

3rd Lecture on Electrochemistry | Chemistry Part I | 12th Std
Notes and Important Points on Electrochemistry - JEE Main 2015

Notes and Important Points on Electrochemistry - JEE Main 2015
Más de Masud Alam Ansari
Más de Masud Alam Ansari (13)
Major Ingredients and Raw materials of Ordinary concrete including Optimum wa...

Major Ingredients and Raw materials of Ordinary concrete including Optimum wa...
Último
Model Call Girl Services in Delhi reach out to us at 🔝 9953056974🔝✔️✔️ Our agency presents a selection of young, charming call girls available for bookings at Oyo Hotels. Experience high-class escort services at pocket-friendly rates, with our female escorts exuding both beauty and a delightful personality, ready to meet your desires. Whether it's Housewives, College girls, Russian girls, Muslim girls, or any other preference, we offer a diverse range of options to cater to your tastes. We provide both in- call and out-call services for your convenience. Our in-call location in Delhi ensures cleanliness, hygiene, and 100% safety, while our out-call services offer doorstep delivery for added ease. We value your time and money, hence we kindly request pic collectors, time-passers, and bargain hunters to refrain from contacting us. Our services feature various packages at competitive rates: One shot: ₹2000/in-call, ₹5000/out-call Two shots with one girl: ₹3500 /in-call, ₱6000/out-call Body to body massage with sex: ₱3000/in-call Full night for one person: ₱7000/in-call, ₱10000/out-call Full night for more than 1 person : Contact us at 🔝 9953056974🔝. for details Operating 24/7, we serve various locations in Delhi, including Green Park, Lajpat Nagar, Saket, and Hauz Khas near metro stations. For premium call girl services in Delhi 🔝 9953056974🔝. Thank you for considering us Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X79953056974 Low Rate Call Girls In Saket, Delhi NCR
Process of Integration the Laser Scan Data into FEA Model and Level 3 Fitness-for-Service Assessment of Critical Assets in Refinery & Process IndustriesFEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads

FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced LoadsArindam Chakraborty, Ph.D., P.E. (CA, TX)
Último (20)
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in South Ex (delhi) call me [🔝9953056974🔝] escort service 24X7
DC MACHINE-Motoring and generation, Armature circuit equation

DC MACHINE-Motoring and generation, Armature circuit equation
Cara Menggugurkan Sperma Yang Masuk Rahim Biyar Tidak Hamil

Cara Menggugurkan Sperma Yang Masuk Rahim Biyar Tidak Hamil
Tamil Call Girls Bhayandar WhatsApp +91-9930687706, Best Service

Tamil Call Girls Bhayandar WhatsApp +91-9930687706, Best Service
Design For Accessibility: Getting it right from the start

Design For Accessibility: Getting it right from the start
S1S2 B.Arch MGU - HOA1&2 Module 3 -Temple Architecture of Kerala.pptx

S1S2 B.Arch MGU - HOA1&2 Module 3 -Temple Architecture of Kerala.pptx
Orlando’s Arnold Palmer Hospital Layout Strategy-1.pptx

Orlando’s Arnold Palmer Hospital Layout Strategy-1.pptx
Kuwait City MTP kit ((+919101817206)) Buy Abortion Pills Kuwait

Kuwait City MTP kit ((+919101817206)) Buy Abortion Pills Kuwait
FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads

FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads
Electrochemistry 1
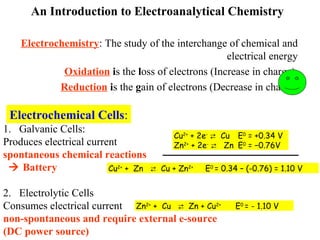
- 1. An Introduction to Electroanalytical Chemistry Electrochemistry: The study of the interchange of chemical and electrical energy Oxidation is the loss of electrons (Increase in charge). Reduction is the gain of electrons (Decrease in charge) Electrochemical Cells: 1. Galvanic Cells: Produces electrical current spontaneous chemical reactions Battery 2. Electrolytic Cells Consumes electrical current non-spontaneous and require external e-source (DC power source) Cu2+ + 2e- Cu E0 = +0.34 V Zn2+ + 2e- Zn E0 = −0.76V Cu2+ + Zn Cu + Zn2+ E0 = 0.34 – (-0.76) = 1.10 V Zn2+ + Cu Zn + Cu2+ E0 = - 1.10 V
- 2. Parts of the voltaic or galvanic cell… Anode the electrode where oxidation occurs After a period of time, the anode may appear to become smaller as it falls into solution. Cathode the anode where reduction occurs After a period of time it may appear larger, due to ions from solution plating onto it. Salt Bridge a device used to maintain electrical neutrality in a galvanic cell This may be filled with agar which contains a neutral salt or it may be replaced with a porous cup. Electron Flow always from anode to cathode (through the wire)
- 4. The diagram to the right illustrates what really happens when a Galvanic cell is constructed from zinc sulfate and copper (II) sulfate using the respective metals as electrodes.
- 5. Electrolysis of molten sodium chloride Electrolytic cells None spontaneous reaction converted to spontaneous reaction Electrical energy converted into chemical energy Conduction in Electrochemical cells: a. External connection Movement of electrons through the external wire. b. Within the solution migration of cations and anions c. At the electrode surface Oxidation/Reduction reaction
- 6. A simplified drawing of a membrane cell for the production of NaOH and Cl2 gas from a saturated, aqueous solution of NaCl (brine).
- 7. Electrolysis from aqueous solution Cathode : 2 competing reactions 2H2O + 2e → H2 + 2OH- Eo = -0.83 V Na+ + e → Na + OH- Eo = -2.71 V H2O with higher red. potential is reduced. Anode : 2 competing reactions 2H2O → O2 + 4H+ + 4e Eo = -1.23 V 2Cl- → Cl2+ 2e Eo = -1.36 V The oxidation pot for H20 is a little higher and since the conc of Cl- is low H2O is oxidized. However, if high conc. Of is used NaCl, due to Consider electrolysis of aqueous NaCl. The process become complex in the presence of water as the water itself can be oxidized or reduced. Cathode – H2 is released Anode – O2 is released for dilute NaCl Cl2 is released for conc. NaCl Experimental results
- 8. Cathodes and Anodes Cathode of an electrochemical cell is the electrode at which reduction occurs Anode of an electrochemical cell is the electrode at which oxidation
- 9. Standard Cell Notation (line notation) • Conventions: Anode on Left Single line : represent phase boundaries Two line : represent liquid junction Cu2+ V NO3 - Zn2+ NO3 - CuZn e- e- Anode / anode solution // cathode solution / Cathode Example: Zn / Zn2+ (1.0 M) // Cu2+ (1.0M) / Cu
- 10. Standard Cell Notation (line notation) • Conventions: Anode on Left Single line : represent phase boundaries Two line : represent liquid junction Cu2+ V NO3 - Zn2+ NO3 - CuZn e- e- Anode / anode solution // cathode solution / Cathode Example: Zn / Zn2+ (1.0 M) // Cu2+ (1.0M) / Cu
