Chemical Reactions
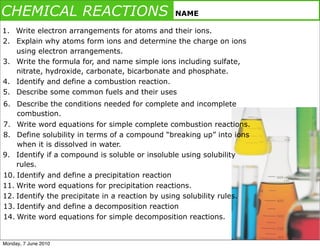
- 1. CHEMICAL REACTIONS NAME 1. Write electron arrangements for atoms and their ions. 2. Explain why atoms form ions and determine the charge on ions using electron arrangements. 3. Write the formula for, and name simple ions including sulfate, nitrate, hydroxide, carbonate, bicarbonate and phosphate. 4. Identify and define a combustion reaction. 5. Describe some common fuels and their uses 6. Describe the conditions needed for complete and incomplete combustion. 7. Write word equations for simple complete combustion reactions. 8. Define solubility in terms of a compound “breaking up” into ions when it is dissolved in water. 9. Identify if a compound is soluble or insoluble using solubility rules. 10. Identify and define a precipitation reaction 11. Write word equations for precipitation reactions. 12. Identify the precipitate in a reaction by using solubility rules. 13. Identify and define a decomposition reaction 14. Write word equations for simple decomposition reactions. Monday, 7 June 2010
- 2. Material World (level 5) • Properties of Materials MW props: Investigate the physical and chemical properties of different groups of substances. For example acids and bases, fuels and metals. Distinguish between pure substances and mixtures and between elements and compounds. • Structure of matter MW struct: Describe the structure of the atoms of different elements. Distinguish between an element and a compound, a pure substance and a mixture at particle level. • Chemistry and society MW soc: Link the properties of different groups of substances to the way they are used in society or occur in nature. Specific Learning Outcome Suggested Learning Activities References Learning to tell the Pretest -> Review the answers. Students will have marked their own work difference between a Web link - on changes of state - use to elicit an understanding of what a physical and chemical physical change is. -> Point out that a Chemical Change is different. Explain reaction. that in a chemcial reaction a new substance is formed, the original substance is used up and the reaction is not reversible. Brainstorm: “Which of these is a chemical reaction?” -> Students write up in booklets Practical: “Physical or Chemical?” Students copy (& complete the method in science books) -> perform & write up the prac. Summary: Students complete the summary (including the brainstorm at the end) -> Review Revision: Recall the 1st Revision: Revise atomic structure (“REVISION”) 20 elements & explain Activity: First run “presenting the elements” with a focus on questioning their position in the whether each element is a metal or a non-metal periodic table --> Colouring in the periodic table. Exercise: Explain the organisation of the periodic table --> “GROUP THERAPY” Exercise: Students list the element names Activity: Learn and recite the poem Flashcards: Students cut out flash cards of the 1st 20 elements and test each other as well as arranging them in order 1st and then arranged to resemble the periodic table. Game: “Element Bingo” Relate Mass & atomic Mass numbers & Atomic numbers -> A different way of showing it numbers to structure, especially electron arrangement Monday, 7 June 2010
- 3. Specific Learning Outcome Suggested Learning Activities References Explain why atoms form Exercise: Explain “Atoms to ions” -> students work through the exercise, ions “IRONING OUT THE IONS” Exercise (done as a class): Explain the organisation of the table of ions. Explain that molecular ions form from groups of atoms that have gained or lost electrons --> “GETTING TO KNOW THE -IDES & -ATES” Exercise (done individually): “-ide or ate?” . Students copy the questions and answer them in their exercise books. Formulae for simple ions Explain that molecular ions form from groups of atoms that have gained or lost electrons --> “GETTING TO KNOW THE -IDES & -ATES” Exercise (done individually): “-ide or ate?” . Students copy the questions and answer them in their exercise books. Identify and define a Reading about Science combustion reaction Practical: “Products of combustion” (lab. 1). Perform -> write up Exercise: Students draw on what they learnt from the Reading about science exercise and the practical to complete the cloze exercise: "What is combustion and what are the products" Describe some common RAS Alert - Demo: The hydrogen bomb - use this demo to point out that in some fuels and their uses combustion reactions carbon dioxide is not produced. A fuel that produces only water as a product is truly the fuel of the future. Demo: “Collecting the products of combustion” - this allows the students to see the products and brings them back to the idea that most fuels will produce Carbon Dioxide and water. Donʼt write this up! Exercise: “Common fuels and their uses” Video: “Free Energy - Turning rubbish into fuel” ----> ---> Discussion: Drive a discussion towards pointing out the virtues of turning rubbish into fuel. Rubbish contains carbon and carbon is everywhere. Ask “Where is the carbon in Questioning: Where is the carbon in this room. -> Eventually it will become rubbish. The process takes the large molecules (all containing carbon) and breaks them down into smaller molecules that burn more easily. Not a very pure fuel but diesel vehicles will run on them. Monday, 7 June 2010
- 4. Specific Learning Outcome Suggested Learning Activities References Describe the conditions Practical: Lab 2 - “Complete & Incomplete combustion” ----> discussion to explain Catalyst 3G: p70, 71 needed for complete and the meaning of complete and incomplete combustion. Why is the flame yellow? incomplete combustion Why is the blue flame clean? Cloze exercise: “Complete & Incomplete combustion” Exercise:: “Global Warming” . Read as a class --> discussion relating to Greenhouse gases. Methane is our biggest emmision. Talk about the Kyoto proticol and carbon credits. Talk about trees as a means of offsetting Carbon DIoxide emissions. Compare NZ (that has to buy credits) to Switzerland that owns carbon credits and explain carbon credit trading. - Students copy & answer Questions 1 to 3 Define solubility in terms Starter: Exercise - “The 3 Sʼs” - this will introduce the students to the terms of a compound breaking “solvent, solute & solution” up into ions. Discussion: With reference to the “SOLUBILITY” notes, define solubility in terms of ions Animation: Show the PHET animation that shows salt breaking up into ions as it is added to water. Exercise (done as a class): “SOLUBLE OR INSOLUBLE?” - Students use the solubility rules to predict the solubility of 6 compounds Identify if a compound is Starter: Sudents need to paste in the lab handout “Just add water!” and predict the http:// soluble or insoluble using solubility of the compounds whose solubility will be tested. www.chem.uiuc.edu/ the solubility rules. Practical: Lab 3 - “Just add water” webfunchem/solubility/ Interactive web activity: Review the results with the help of the chem.uiuc SolExp.htm website (opposite) Identify and define a Starter: Word searches: Hwk.1 & 2 -> finish 4 hwk. These have the words that will precipitate reaction be tested in the language test Theory: "WHAT IS A PRECIPITATE?" -> "WORD EQUATIONS FOR Write word equations for PRECIPITATE REACTIONS" precipitation reactions Practical: Lab 4: "ALL MIXED UP" Copy (if time) -> Perform -> Review Identify a precipitate in a reaction by using the solubility rules Identify and define a Starter: Crossword Puzzles: Hwk.3 & 4 -> finish 4 hwk. These have the meanings decomposition reaction that will be tested in the language test. RAS Alert: Aiming to introduce the importance of decomposition reactions. (i) Write word equations for Baking ... “My daughter bakes ... Baking soda ... decomposition” (ii) “At the simple decomposition hairdresser .... Peroxide” (iii) Limestone decomposition through volcanic activity -> reactions Carbon Dioxide -> Plant life -> Oxygen production -> Life on earth” Theory: “DECOMPOSITION REACTIONS” Practical: Lab 7 - Heating Copper Carbonate Monday, 7 June 2010 Demo: Demo 2 - “THE DECOMPOSITION OF HYDROGEN PEROXIDE”
- 5. Specific Learning Outcome Suggested Learning Activities References Identify and define a Starter: Crossword Puzzles: Hwk.3 & 4 -> finish 4 hwk. These have the meanings decomposition reaction that will be tested in the language test. RAS Alert: Aiming to introduce the importance of decomposition reactions. (i) Write word equations for Baking ... “My daughter bakes ... Baking soda ... decomposition” (ii) “At the simple decomposition hairdresser .... Peroxide” (iii) Limestone decomposition through volcanic activity -> reactions Carbon Dioxide -> Plant life -> Oxygen production -> Life on earth” Theory: “DECOMPOSITION REACTIONS” Practical: Lab 7 - Heating Copper Carbonate Demo: Demo 2 - “THE DECOMPOSITION OF HYDROGEN PEROXIDE” REVISION literacy test --> targetted revision: Questions on the board that mimic the test questions. TEST Monday, 7 June 2010
- 6. GLOSSARY Monday, 7 June 2010
- 7. Hwk 1/exercise WORD SEARCH - 1 Word List U R A X P X J V A J J B Z E T proton I O N I C I W S N P O F L G Y neutron F P M M H H H T Q B R E C U J electron nucleus V V A B A N R S P R M F I K X element U S W S T I N O I E L E M C N atom S N O T O R P E N I N N O E V ion W U A C M C D T U D L H T H U ionic cation F C A V G K D H R T C M A G R anion B L K N M O E A W U R S Z E L atomic number W E E V I N J B B H E O B F A mass number D U L F X O O F N P G M N S M Q S T W M J N I I S U G H C E E L E C T R O N T N W U Z K Q I B J O N V O U O A W I H A T X Y V F H W H M T U C S Y G W Monday, 7 June 2010
- 8. Hwk 2 WORD SEARCH - 2 D N G N X F M N W C S B N P A Word List F Y O D Z X T S O O J O T R L formula Z J X I H V J M Z A I N V E U configuration S D B I T Y B L U T E Z F C M combustion hydrocarbon A L C C B U D K A V D E H I R solute M S K C S R L R L N C B I P O solvent J J V T K C U O O J J F V I F solution K G I Z K G S F S C K E J T X solubility U O S U I V V N P R A P M A D precipitate decomposition N C N F S O L U T E J R V T I G G N G C W A M X D I M B E L H O D E C O M P O S I T I O N C Y T I L I B U L O S P V J N W F Y B V K F A A V K H R R S C L D M V L T R F U O G W Z V Monday, 7 June 2010
- 9. Term Definition GLOSSARY 1 Match the term with its definition > ans’s only in BOB A. Atom 1. A code that shows the number and type of each element in a compound B. Element 2. This is another word for arrangement C. Proton 3. The building blocks of matter. D. Neutron 4. A substance that contains one type of atom only 5. A particle within the nucleus of an atom that has mass and is positively E. Nucleus charged 6. A particle within the nucleus of an atom that has mass and has no F. Electron electrical charge G. Electron 7. A number which represents the number of protons in the nucleus of an shell atom (also equal to the number of electrons) H. Formula 8. An energy level around the nucleus of an atom I. Configuration 9. A negatively charged ion J. Atomic 10. A negatively charged particle surrounding the nucleus of an atom number K. Mass 11. A positively charged ion number L. Ion 12. The central, dense positively charged part of an atom 13. A mixture of an insoluble substance with a liquid. The insoluble substance M. Ionic eventually settles. N. Cation 14. The number of particles (protons & neutrons) in the nucleus O. Anion 15. An atom that has lost or gained electrons P. Suspension 16. A word used to describe a substance that is made up of ions Monday, 7 June 2010
- 10. Term Definition GLOSSARY 1- HANDOUT Atom The building block of matter. Element A substance that contains one type of atom only Proton A particle within the nucleus of an atom that has mass and is positively charged A particle within the nucleus of an atom that has mass and has no electrical Neutron charge Nucleus The central, dense positively charged part of an atom Electron A negatively charged particle surrounding the nucleus of an atom Electron shell An energy level around the nucleus of an atom Formula A code that shows the number and type of each element in a compound Configuration This is another word for arrangement A number which represents the number of protons in the nucleus of an atom Atomic number (also equal to the number of electrons) Mass number The number of particles (protons & neutrons) in the nucleus Ion An atom that has lost or gained electrons Ionic A word used to describe a substance that is made up of ions Cation A positively charged ion Anion A negatively charged ion A mixture of an insoluble substance with a liquid. The insoluble substance Suspension eventually settles. Monday, 7 June 2010
- 11. Term Definition GLOSSARY 2 Match the term with its definition > ans’s only in BOB A. Fuel 1. The solid that is dissolved B. Hydrocarbon 2. The liquid that does the dissolving C. Combustion 3. Able to be dissolved D. Carbon 4. a mixture where very small particles are evenly spread through the dioxide liquid particles (the small particles don't settle) E. Carbon 5. When an ionic compound placed in water has broken up and individual monoxide particles are too small to be seen F. Soot 6. A deadly gas given off when fuels burn in a low oxygen environment 7. Another word for carbon that is produced when a fuel is burnt in a low G. Dissolved oxygen environment H. Solute 8. A substance that can combine with oxygen to provide heat energy 9. A compound that consists of Hydrogen and Carbon atoms only (often I. Solvent a fuel) 10.A chemical reaction which involves a substance combining with J. Solution oxygen K. Soluble 11.A solid product formed when two solutions react together L. Solubility 12.The breakdown of a substance into simpler substances M. Insoluble 13.The degree to which a substance will dissolve N. Precipitate 14.A gas formed as a result of a fuel burnt in a high oxygen environment O. Decomposition 15.Not able to be dissolved Monday, 7 June 2010
- 12. Term Definition GLOSSARY 2- HANDOUT Fuel A substance that can combine with oxygen to provide heat energy Hydrocarbon A compound that consists of Hydrogen and Oxygen atoms only (often a fuel) Combustion A chemical reaction which involves a substance combining with oxygen Carbon dioxide A gas formed as a result of a fuel burnt in a high oxygen environment Carbon monoxide A deadly gas given off when fuels burn in a low oxygen environment Another word for carbon that is produced when a fuel is burnt in a low Soot oxygen environment When an ionic compound placed in water has broken up and individual Dissolved particles are too small to be seen Solute The solid that is dissolved Solvent The liquid that does the dissolving a mixture where very small particles are evenly spread through the liquid Solution particles (the small particles don't settle) Soluble Able to be dissolved Solubility The degree to which a substance will dissolve Insoluble Not able to be dissolved Precipitate A solid product formed when two solutions react together Decomposition The breakdown of a substance into simpler substances Monday, 7 June 2010
- 13. INTRO Monday, 7 June 2010
- 14. PRE-TEST NAME 1. True or False? (a) Matter is made up of tiny particles called atoms. (b) An element can contain more than one type of atom. (c) The periodic table does not contain all the elements. (d) Melting requires a solid to cool. (e) A solution is an example of a mixture. 2. There are two types of changes that occur in chemistry - physical and chemical. Which of the following is an example of a physical reaction? (Circle the answers) A. Clouds forming B. Leaves drying out C. Wood burning D. Glass melting 3. What are the 3 states of matter? ____________, ____________ & ____________ 4. Use the words below to complete the following sentences: When substances change state the _____________ and __________ of the particles changes but the ___________ of particles remains the same. This is an example of a _______________ reaction. ____ new substance is formed. Word List type no arrangement physical motion http://www.harcourtschool.com/activity/ states_of_matter/ Monday, 7 June 2010
- 15. /10 ANSWERS TO PRE-TEST NAME 1. True or False? (a) Matter is made up of tiny particles called atoms. T (b) An element can contain more than one type of atom. F (c) The periodic table does not contain all the elements. F 5 marks (d) Melting requires a solid to cool. F (e) A solution is an example of a mixture. T 2. There are two types of changes that occur in chemistry - physical and chemical. Which of the following is an example of a physical reaction? (Circle the answers) A. Clouds forming B. Leaves drying out 3 marks C. Wood burning D. Glass melting 1 mark Solid Liquid Gas 3. What are the 3 states of matter? ____________, ____________ & ____________ 4. Use the words below to complete the following sentences: arrangement motion When substances change state the _____________ and __________ of the types particles changes but the ___________ of particles remains the same. This is an physical example of a _______________ reaction. ____ new substance is formed. No 1 mark Word List types no arrangement physical motion http://www.harcourtschool.com/activity/ states_of_matter/ Monday, 7 June 2010
- 16. WHICH OF THESE IS A CHEMICAL REACTION? ..... AND WHY? Answer these questions as full sentences. _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ A bus rusting _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ Polar ice melting _____________________________________ Monday, 7 June 2010
- 17. _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ Water boiling _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ _____________________________________ A nuclear explosion Monday, 7 June 2010
- 18. Activities: Burning Magnesium (heat given off) Magnesium ribbon+ Hydrochloric acid Copper sulphate + zinc powder (colour change) Heating nichrome wire Heating candle wax Ammonium dichromate - demo Iodine sublimation - loosely fitting stopper on test tube - demo A CHEMICAL REACTION IN CONTEXT Why does it start? Why does in stop? Monday, 7 June 2010
- 19. HEATING MAGNESIUM RIBBON Tongs Observation Magnesium ribbon Bunsen burner Mat Chemical or physical? HEATING NICHROME WIRE Observation Tongs Nichrome wire Bunsen burner Mat Chemical or physical? Monday, 7 June 2010
- 20. HEATING CANDLE WAX Candle wax Observation Evaporating basin xxxxxxxxxx Blue bunsen flame Chemical or physical? COPPER SULPHATE & ZINC POWDER Observation Zinc powder Chemical or physical? Copper sulphate Shake from side to side by tapping with your finger (for a few minutes) Monday, 7 June 2010
- 21. MAGNESIUM RIBBON + HYDROCHLORIC ACID Magnesium Observation ribbon (0.5 cm) Hydrochloric acid (1 cm) Chemical or physical? Demonstrations HEATING AMMONIUM DICHROMATE HEATING IODINE Monday, 7 June 2010
- 22. SUMMARY Word List There are two types of change that we commonly see: new 1. ______________ change 2. _______________ change original physical In a chemical change: chemical • A ________substance is formed • The ____________ substance is used up recover • It is not possible to __________ to the original substance Observations that we might make if a chemical reaction has occurred: 1. Fizzing (gas produced) 2. Colour change Copy 3. Heat or cold into notes 4. Smell In a physical change: • No new substance is formed • The original substance is not used up Monday, 7 June 2010
- 23. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 24. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 25. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 26. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 27. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 28. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 29. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 30. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 31. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 32. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 33. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 34. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 35. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 36. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 37. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 38. 1. Cut out the terms and place them into your mind map 2. Once your work has been checked, paste them in No new substance Magnesium & Hydrochloric acid (fizzing) Boiling water Heating iodine Original substance used up Not reversible New substance formed Reversible Heating Ammonium Chemical Dichromate (Heat) Melting wax Physical Copper sulphate and zinc Heating wire (Colour change) Monday, 7 June 2010
- 39. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 40. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 41. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 42. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 43. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 44. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 45. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 46. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 47. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 48. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 49. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 50. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 51. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 52. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 53. MIND MAP Boiling water No new substance Heating wire Physical Examples Reversible Heating iodine Types of change Melting wax New substance formed Chemical Original substance used up Not reversible Examples Magnesium & Copper sulphate Heating Hydrochloric and zinc Ammonium acid (fizzing) (Colour change) Dichromate (Heat) Monday, 7 June 2010
- 54. THE ELEMENTS Monday, 7 June 2010
- 55. Copy ELEMENTS Complete the sentences, table and diagram (below) Elements are like the letters of the alphabet. There are 26 letters and these can be joined together in different ways to make up to 750 000 different words. Letters cannot be split into anything simpler. • Elements are substances that contain particles called • An element consists of only one type of atom only. • Atoms cannot be easily into anything . • There are 90 different elements and these can be . to make all the other in the world Atoms Part of Where Electrical atom found Charge Proton + Neutron + Electron Proton Word list: substances, atoms, simpler, split, joined, proton, electron, empty space, negative, positive, neutral, neutron, in the “History of the atom” Activity: Mystery Object nucleus, outside the nucleus. Monday, 7 June 2010
- 56. Getting to know the periodic table http://www.webelements.com/ Shade in the periodic table using the key (below) x x x x x x x x x x Blue: Metals Green: Non-metals Key The elements Inert gases: Yellow song! Black zig zag line: separates the metals from non-metals Monday, 7 June 2010
- 57. PR TH ES EL E EN EM TI EN N G TS Monday, 7 June 2010
- 58. Monday, 7 June 2010
- 59. Monday, 7 June 2010
- 60. Monday, 7 June 2010
- 61. Monday, 7 June 2010
- 62. Monday, 7 June 2010
- 63. Monday, 7 June 2010
- 64. Monday, 7 June 2010
- 65. Monday, 7 June 2010
- 66. Monday, 7 June 2010
- 67. Monday, 7 June 2010
- 68. HYDROGEN Monday, 7 June 2010
- 70. Hg Monday, 7 June 2010
- 71. Al http://sam.davyson.com/as/physics/aluminium/site/uses.html Monday, 7 June 2010
- 72. Monday, 7 June 2010
- 73. Monday, 7 June 2010
- 74. HISTORY of aluminium Monday, 7 June 2010
- 75. HISTORY of aluminium 5300 BC. ancient Persia Egyptians and Babylonians around 4000 years ago as fabric dyes and cosmetics. Monday, 7 June 2010
- 76. WEAKNESSES Monday, 7 June 2010
- 77. Hydrogen - not a metal THE PERIODIC TABLE but here because of its electron arrangement 1 2 3 4 5 6 7 8 Non-metals Less reactive More reactive metals Inert gases metals Monday, 7 June 2010
- 78. ORGANISATION Copy Atomic number Atomic numbers are the smaller of the two numbers associated with each element. Atomic numbers increase by one from left to right of the table Rows The atoms get larger in size from left to right across a row as their mass increases Columns The atoms get larger in size and increase in mass from top to bottom of a column. Elements in a column have similar properties. Columns are often called groups. Groups start at 1 (at the left) and finish with group 8 (at the right of the table) Write the group numbers into your table GROUP THERAPY Complete the table (below) Group Element Symbol Atomic number Chlorine Potassium Magnesium Oxygen Monday, 7 June 2010
- 79. Monday, 7 June 2010
- 80. Element names H Na He Mg Li Al Be Si B P C S N Cl O Ar F K Ne Ca An easy way to remember the first 20 elements Flashcards (in exercises) Harry He Likes Beer By Cupfuls Not Over Flowing Never Natter Magic Although Science Possesses Some Clues Arthur Kicks Cats “Element Bingo” (in starters) Monday, 7 June 2010
- 81. ELEMENTS, MIXTURE AND COMPOUND Aim to investigate the product of a zinc reacting with sulphur. Heating a mixture of zinc Mixture of zinc powder powder and sulphur powder and sulphur powder Bottle cap Keep a safe distance xxxxxxxxxx away from the experiment !! Blue bunsen flame Appearance of the elements before heating: Iron Sulphur Appearance of the compound formed after heating: Name of compound formed: ________________________ Monday, 7 June 2010
- 82. IT’S ELEMENTARY Name 1. What is the name of the particle that cannot easily be made smaller? _____________________ 2. Name 2 objects in the room that contain carbon. ________ , __________ 3. Air is made up mainly of which element? _________ 4. Explain the difference between an element and a compound. ________________________________________________ ________________________________________________ 5. Write the symbols for the following elements: (a) Lithium ____ (b) Boron ____ (c) Fluorine ____ (d) Argon (e) Silicon ____ (f) Phosphorus _____ (g) Chlorine ____ (h) Sodium _____ (i) Potassium ____ 6. Name the following elements: (a) H __________(b) He __________ (c) Be __________ (d) C __________ (e) N __________ (f) O __________ (g) Ne __________ (h) Mg __________ (i) Al __________ (j) S __________(k) Ca __________ Monday, 7 June 2010
- 83. ELEMENTS, MIXTURE AND COMPOUND Demo: Iron heated with sulphur test-tube mixture of powdered sulphur and iron Bunsen burner (blue flame) Appearance of the elements before heating: Iron Sulphur Appearance of the compound formed after heating: Name of compound formed: ________________________ Monday, 7 June 2010
- 84. ELEMENT CUBES Each side of the cube: 1. Name & symbol of element 2. Uses of the element 3. Classification (metal/non-metal) 4. Physical properties (colour, s/l/g) 5. History - discovery 6. Extraction Each student allocated a different element. Put into letter box. Reallocate. Research -> element cube (book computers, finish for Hwk) Monday, 7 June 2010
- 85. HI ST OF O TH RY AT E OM Monday, 7 June 2010
- 86. DISCOVERING ATOMS - MISSION IMPOSSIBLE?? Monday, 7 June 2010
- 87. Practical HOW HAVE WE LEARNT THIS Mystery object inside My lab group matchbox passed from person to person Bart match box Maggie Lisa Without opening the box, each person makes an observation Monday, 7 June 2010
- 88. Our groups results Observation Observation Observation D Bart I S C U Lisa S S I Maggie O N We think the object in the container is _____________ ___________________________________________ Monday, 7 June 2010
- 89. Dalton, John (b. Sept. 6, 1766, Eaglesfield, Cumberland. Eng.- d. July 27, 1844, Manchester) “one of the fathers of modern physical science” “... a teacher of mathematics and natural philosophy ...” “ ... a chemist and a physicist ...” 1803 • Elements consist of identical atoms • Atoms are like dense spheres • Atoms are indestructible Monday, 7 June 2010
- 90. Born on December 18, 1856 near Manchester, England The young Thomson was chosen to be the third Cavendish Professor in 1884 In 1906 he was awarded the Nobel prize in physics ... “electrical discharge in gases” 1897 • “Plum Pudding Model” an atom consists of a sphere of positive charges with negative charges distributed evenly through + + - the sphere): + - + - - + + + - Listen to JJ (on mp3): http://www.aip.org/history/ electron/jjsound.htm "Could anything at first sight seem more impractical than ...” Monday, 7 June 2010
- 91. born on August 30, 1871, in Nelson, New Zealand He graduated with an M.A. from the university in Wellington in 1893 with a double major in Mathematics and Physical Science In 1894, he was awarded an 1851 Exhibition Science Scholarship, enabling him to go to Trinity College, Cambridge, as a research student at the Cavendish Laboratory under J.J. Thomson. 1911 Rutherford performs his famous “Gold foil experiment”. Most of an atom is made up of empty space. 1912: Rutherford and Neils Bohr join forces. The quantum theory is incorporated into Rutherford’s model > > > > > The model that is still currently used. Monday, 7 June 2010
- 92. Monday, 7 June 2010
- 93. ATOMIC STRUCTURE Monday, 7 June 2010
- 94. MASS NUMBERS & ATOMIC NUMBERS An element in the periodic table is described like this: The mass number is 19. 19 The number of protons plus neutrons =19 9 F Fluorine The atomic number is 9. There are 9 protons in the nucleus and 9 electrons around it In this example: The 19 particles in the nucleus are protons or neutrons I’m lost! 9 of these particles are protons therefore there are 10 neutrons in the nucleus Summary For an atom: • The atomic number gives the number of protons • The atomic number is also gives the number of electrons • The mass number is the number of protons plus neutrons • neutron number = mass number - atomic number Monday, 7 June 2010
- 95. ATOMIC NUMBERS & MASS NUMBERS The mass number is the number of particles in the nucleus 19 9 F Fluorine The atomic number is the number of protons in the nucleus The mass number is ____ so there are _____ particles in the nucleus. The atomic number is ___ so _____ of them are protons Therefore the rest of them are neutrons so there are 10 neutrons number of Symbol of element number of protons number of neutrons electrons 19 F 9 11 B 5 16 O 8 28 Si 14 35 Cl 20 31 P 15 Monday, 7 June 2010
- 96. Individual Exercise TRY THIS! number of Symbol of element number of protons number of neutrons electrons 9 Be 4 21 Ne 10 27 Al 13 39 K 20 15P 16 42 Ca 20 12 C 6 7 Li 3 23 Na 11 24 Mg 12 14 N 7 16S 16 Monday, 7 June 2010
- 97. ELECTRON ARRANGEMENTS Monday, 7 June 2010
- 98. ELECTRON ARRANGEMENTS Electrons in the electron cloud are not arranged randomly around the nucleus. • Those close to the nucleus have low energy • Those far away from the nucleus have high energy Copy Electrons are arranged in energy levels For the 1st 20 elements there are 4 energy levels: Level 1 can hold a maximum of 2 electrons Level 2 “ “ “ “ “ 8 electrons Level 3 “ “ “ “ “ 8 electrons Level 4 “ “ “ “ “ 2 electrons Example 1 40 20 protons in the nucleus 20 Ca (the atomic number) Calcium => 20 electrons around the 2.8.8.2 nucleus Electron arrangement: “ 2 in the 1st shell, 8 in the 2nd shell, ....... Monday, 7 June 2010
- 99. CONFIGURE THIS Use your knowledge of electron arrangement to complete the table below. The electron 1 arrangements are shown below H 1 the element names Hydrogen 1 4 2 He Helium 2 7 9 11 12 14 16 19 20 3 Li 4 Be 5 B 6 C 7 N 8 O 9 F 10 Ne Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 2.1 2.2 2.3 2.4 23 24 27 28 31 32 37 40 11 Na 12 Mg 13 Al 14 Si P S Cl Ar Sodium Magnesium Aluminium Silicon Phosphorus Sulphur Chlorine Argon 2.8.1 2.8.2 2.8.3 39 40 K Ca Potassium Calcium Monday, 7 June 2010
- 100. Copy all SHELL DIAGRAMS Electrons fill up an atoms shell in order, first they fill up the inner shell (first electron shell) then the next shell and so on An exercise done as a class: Process: 40 x 1. Use your periodic table to find the atomic Ca x x x x x number. 20 x x x x x x 2. The atomic number will tell you how Calcium x x x many electrons there are x x x x x 3. Fill the electrons according to the rule: Level 1 can hold a maximum of 2 electrons Example 2 - Silicon Level 2 “ “ “ “ “ 8 electrons Level 3 “ “ “ “ “ 8 electrons Level 4 “ “ “ “ “ 2 electrons 28 14 Si 4. Start filling the levels from level 1. 5. When level 1 is full start filling level 2. When Silicon level 2 is full start filling level 3 and so on. 6. Stop filling the levels when you have used all the electrons that the atom has. Monday, 7 June 2010
- 101. Individual Exercise A DIFFERENT WAY OF SHOWING IT Example: Fluorine, 9 19F has 9 electrons x For each of the following atoms use your periodic table to find the number of x x x electrons, draw the electron shell diagram, showing the nucleus as a solid circle. A second example x x 1. 13 27Al x 2. 11 23Na x x x x x x x x x x x x x x x 3. 14 28Si 4. 15 31P 5. 16 32S Homework (in science books): (i) Be (ii) F (iii) Ne (iv) Ar Finish for homework Monday, 7 June 2010
- 102. Monday, 7 June 2010
- 103. Monday, 7 June 2010
- 104. Monday, 7 June 2010
- 105. ATOMS TO IONS Monday, 7 June 2010
- 106. ATOMS TO IONS Copy An ion is an atom that has lost or gained electrons “Ions are more stable than atoms. IONS HAVE AN OUTER SHELL THAT IS FULL... Using this knowledge it is possible to work out the arrangement of electrons in ions.” Sodium atom --> Sodium ion 1 electron E x x x x x lost x X x x x x x x x x A x x x x x x x 1+ charge M Na Na+ P Chlorine atom --> Chloride ion 1 electron L x x x x x x x x x gained x x x x x E x x x x x x x x x x x S x x x x x x x x x x Cl Cl- 1- charge Monday, 7 June 2010
- 107. IRONING OUT THE IONS Copy “Metal atoms lose electrons. Non-metal atoms gain electrons. No more than 3 electrons can be lost or gained” Copy & complete the following table showing the electron arrangements of the atoms and their ions: Metal atom Metal ion Non-metal atom Non-metal ion C: 2, 4 no ion formed Cl: 2, 8, 7 Cl-: 2, 8, 8 Mg: 2, 8, 2 Mg2+: 2, 8 N: 2, 5 N3-: 2, 8 Li: 2, 1 Li+: 2 Ar: 2, 8, 8 No ion formed Be O Ca S Al F Na P Draw small Beryllium Sulphide Aluminium shell diagrams for the following ions Monday, 7 June 2010
- 108. FORMULAE FOR SIMPLE IONS Background A chemical formula shows how atoms or ions are joined to make compounds. (A compound consists of two or more different atoms that are joined chemically). An ionic compound is formed when positive and negative ions are attracted to each other. Some ions comprise groups of atoms that have gained or lost electrons. These groups are the “-ides” or “-ates”. A table of common ions is shown below: +1 +2 +3 _,, -1 H* Mg2* Al3+ c1- gz- hydrogen magnesium aluminium chloride oxide Li* Ca2* Fe3* oH- COr'- lithium calcium iron(III) hydroxide carbonate Na+ Fe2* No,* Soo'- sodium iron(II) nitrate sulfate K+ Cu2* HCO3- PO43- potasslum copper(II) hydrogen carbonate Phosphate Zn2* zinc Pb2+ lead Monday, 7 June 2010
- 109. GETTING TO KNOW THE “-IDES” AND “-ATES” Complete the columns by writing the “words” -ide or -ate “-ates” end in O4 and O3 the rest are “-ides” An exercise done as a class: + ion - ion Formula -ide/-ate + ion - ron Formula -ide/-ate Znzr N3- ZneNz Ca2* NOs- Ca(NOs)z Pb2* Br PbBrz Fe3* SO+2- Fe2(S04)3 I Ag* s2- Li* COs2- LizCOs ) Fe3* cl- 6 Ag* PO+3- 3 Na* 02- 7 NH+* SO+2- K+ t- KI 8 Pbz* COs2- Cu2* SO+2- CuSOa 9 Al3* oH- 4 Mgz+ COs2- 10 Cu2* s2- 5 Zn2* 02- 11 (+ HCOg- Exercises: “-ide or -ate” Monday, 7 June 2010
- 110. FUELS Monday, 7 June 2010
- 111. DA BOMB flame Hydrogen gas Open end for air to enter Clamp stand Monday, 7 June 2010
- 112. DO YOU KNOW?..... “What is combustion??” .... And what are the products? Monday, 7 June 2010
- 113. PRODUCTS OF COMBUSTION Aim: to investigate the products of combustion Beaker Blue cobalt chloride paper taped to the inside of the beaker vial Bunsen mat limewater Candle Observations Conclusion Monday, 7 June 2010
- 114. Reading WHAT IS A COMBUSTION REACTION? about Science Common fuels will usually contain carbon and hydrogen. This is why they are called hydrocarbons. Some fuels contain more carbon than others. The carbon content of fuels gives us an idea of how much heat energy is produced when the fuel is burnt. Carbon in the fuel combines with oxygen to produce carbon dioxide. This occurs when there is plenty of oxygen. When oxygen is in short supply, carbon monoxide is formed. Carbon dioxide molecules each have an atom of carbon joined to two atoms of oxygen but carbon monoxide molecules have only one oxygen atom joined to each carbon atom. So carbon monoxide is formed when there is not enough oxygen to form carbon dioxide. Petrol is a very popular fuel. It is used in most car engines. Unfortunately the car engine is not very efficient. Only about half of the energy stored in petrol is changed to energy of movement. One reason for this is that the petrol does not combine with enough oxygen as it burns. This means that some carbon monoxide is produced as well as carbon dioxide. Really old cars will also produce some soot, which is really just carbon. Getting more oxygen mixed in with the petrol before it gets into the engine’s cylinders will produce hotter and cleaner combustion in the engine. So the amount of oxygen available will also have an effect on how much heat is produced when a fuel burns and how clean the flame will be. To most people’s surprise, water is also a product of combustion or burning. It is possible to see that water is produced from the burning of petrol by looking at the end of your car’s muffler on a cold morning while your car is idling in your driveway. Droplets of water will drip from the end of the muffler and form a small puddle on your driveway. Monday, 7 June 2010
- 115. COMBUSTION & FUELS Copy Combustion (or burning) occurs when a fuel combines with ______________ in a chemical reaction. Starting a fire Some heat energy is needed to get chemical reaction going. This is why a fuel often needs a match to get it to burn. Keeping a fire going Once a fire has started it is the heat from the fire that keeps this chemical reaction going. Putting out a fire • using water - this cools it down so that there is not enough heat to keep the chemical reaction going. • smothering it with a blanket or with Carbon Dioxide - this prevents it from getting oxygen Most fuels contain _____________ and _____________ and so are called hydrocarbons. Carbon atoms in a hydrocarbon join with oxygen to make gases like _______________ and ______________ . ______________ ________________ turns limewater milky. ____________ turns blue cobalt chloride paper pink. Monday, 7 June 2010
- 116. PUTTING OUT A FIRE WITH A BOMB Monday, 7 June 2010
- 117. WATER PUTS OUT FIRES BECAUSE IT COOLS THEM DOWN Monday, 7 June 2010
- 118. PUTTING OUT FIRES Vin ega r 250 mL beaker candles (of different lengths placed into film cannisters) baking soda Observations Explanation Monday, 7 June 2010
- 119. FREE ENERGY - TURNING RUBBISH INTO FUEL Monday, 7 June 2010
- 120. Monday, 7 June 2010
- 121. Monday, 7 June 2010
- 122. COMPLETE AND INCOMPLETE COMBUSTION Copy Complete combustion - occurs when there is plenty of oxygen When combustion is complete, the only products are Carbon dioxide and water. A general equation for this reaction is: Fuel + + Hot, clean, blue flame that is difficult to see. Incomplete combustion - occurs when oxygen is in short supply Some carbon monoxide will also be formed and if oxygen is very scarce there will also be some carbon (soot) produced. Dirty, yellow flame. Not good for heating but difficult to see. Fuel + + Exercises: “Global Warming” Catalyst 3G Copy and answer Q.1 to 3 Monday, 7 June 2010
- 123. SOLUBILITY Monday, 7 June 2010
- 124. SOLUBILITY Solvent Negative ion Water - + molecules + - + Solution Solute -+ - + particle - - + + - + Positive Mixed up solid and ion liquid particles The sorts of compounds that dissolve easily in water are compounds that contain positive and negative ions, joined together (ionic compounds). Dissolving occurs when the compound breaks up into its ions and these ions spread evenly amongst the water molecules. The ability of a substance to dissolve is called its solubility. http://phet.colorado.edu/simulations/sims.php?sim=Salts_and_Solubility Monday, 7 June 2010