001 carnot cycle
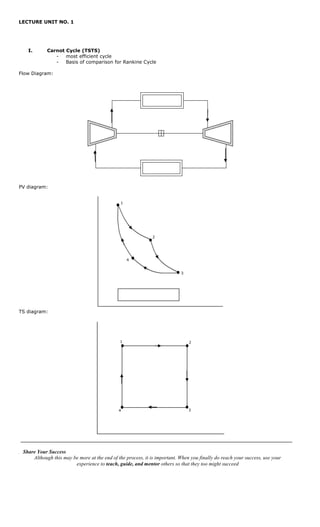
- 1. LECTURE UNIT NO. 1 I. Carnot Cycle (TSTS) - most efficient cycle - Basis of comparison for Rankine Cycle Flow Diagram: PV diagram: 1 2 4 3 TS diagram: 1 2 4 3 . Share Your Success Although this may be more at the end of the process, it is important. When you finally do reach your success, use your experience to teach, guide, and mentor others so that they too might succeed
- 2. DEFINITIONS: During a certain process Expansion Ratio: re = Vmax / Vmin Compression Ratio: rk = Vmax / Vmin Cut off Ratio: rc = Vmax / Vmin Pressure Ratio: rp = Pmax / Pmin EQUATIONS: 1. PVT Relationships: Process: 1 – 2 Isothermal Heat Addition Process (T = C) P2V2 / T2 = P1V1 / T1 V2 / V1 = P1 / P2 = re (T=C) Process: 2 - 3 Isentropic Expansion Process (S = C) T3 / T2 = (P3 / P2) k-1 / k = (V2 / V3) k-1 (T3 / T2) 1/ k-1 = (P3 / P2) 1 / k = V2 / V3 (T2 / T3) 1/ k-1 = (P2 / P3) 1 / k = V3 / V2 = re (S=C) Process: 3 - 4 Isothermal Heat Rejection Process (T = C) P4V4 / T4 = P3V3 / T3 V3 / V4 = P4 / P1 = rk (T=C) Process: 4 – 1 Isentropic Compression Process (S = C) T1 / T4 = (P1 / P4) k-1 / k = (V4 / V1) k-1 (T1 / T4) 1/ k-1 = (P1 / P4) 1 / k = V4 / V1 = rk (S=C) NOTE: A. Isentropic expansion ratio = Isentropic compression ratio re (S=C) = rk (S=C) V3 / V2 = V4 / V1 B. Isothermal expansion ratio = Isothermal compression ratio re (T=C) = rk (T=C) V2 / V1 = V3 / V4 Hence: P1 / P2 = V2 / V1 = V3 / V4 = P4 / P3 2. Heat Added, QA = Σ + Q Process: 1 - 2 (T=C) Q1-2 = P1V1 ln V2 / V1 = P2V2 ln V2 / V1 Seek Input Whatever your idea of success, conduct a "sanity check" throughout the process of reaching your goal. This should be done with someone you trust and who is themselves successful. Ask them to provide honest feedback about your success and as you move through different milestones, bounce concerns or new ideas off them to help keep you on the right track.
- 3. 3. Heat Rejected, QR = Σ - Q Process: 3 – 4 (T=C) Q3-4 = P3V3 ln V4 / V3 = P4V4 ln V4 / V3 4. Network, Wnet Wnet = | QA | - | QR | = | mRT1 ln P1 / P2 | - | - mRT4 ln P1 / P2 | Wnet = mR(T1 – T4) ln P1 / P2 5. Carnot Cycle Thermal Efficiency, ecc ecc = Wnet / QA x 100 % = mR(T1 – T4) ln P1 / P2 x 100 % mRT1 ln P1 / P2 = T1 – T4 x 100 % T1 ecc = TH – TL x 100 % TH 6. Carnot Cycle Mean Effective Pressure, Pm Pm = Wnet / VD = Wnet / (V3 – V1) Pm = ecc x QA / (V3 – V1) SEATWORK: 1. A Carnot Cycle engines uses 0.5 kg/s of air as the working substance. The pressure and temperature at the start of isothermal expansion are 1 MPa and 370 °C respectively. If the isothermal expansion ratio is 4 and the adiabatic expansion ratio is 2, determine: a. The heat added ans. 127.91 kJ/s b. The heat rejected ans. -96.94 kJ/kg c. The thermal efficiency ans. 24.21 % d. The mean effective pressure ans. 47.94 kPa 2. An air-standard Carnot Cycle is executed in a closed system between the temperature limits of 350 and 1200 K. The pressures before and after the isothermal compression are 150 kPa and 300 kPa, respectively. If the network output per cycle is 400 kJ, determine: a. The maximum pressure in the cycle ans. 22388.11 kPa b. The heat transfer in air, QA ans. 564.71 kJ c. The mass of air ans. 2.37 kg Note: Use Rair = 0.287 kJ/kg-K k = 1.4 if not given in the problem No Shortcuts An old cliché states, "Anything worth doing is worth doing well." This should be your motto. When you want to succeed, you cannot afford to take shortcuts. Taking shortcuts leads to imperfection and inadequacies. Always strive for the best, even if it requires a little more time and effort.