Sea and Seawater
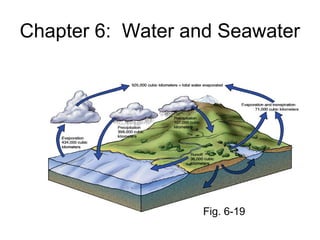
- 1. Chapter 6: Water and Seawater Fig. 6-19
- 2. Atomic structure Atoms: • Building blocks of all matter Structure: • Nucleus (nucleos = little nut) Inside of the Nucleus: • Protons (+ charge) and neutrons (no charge) Surrounding Nucleus: • Electrons (- charge) • Ions are charged atoms and can be lost or gained
- 3. Protons • The number of protons is what distinguishes atoms of the 115 known chemical elements. Example: • An atom of Oxygen (O) has 8 protons, no other element will have 8 protons. • An atom of Hydrogen (H) has only 1 proton and an atom of Helium (He) has only two protons
- 4. Water molecule Molecule (molecula = a mass): group of two or more atoms Water Molecule (H2O) • Two hydrogen, one oxygen • Covalent Bond (bonded by sharing electrons) • Bend in geometry creates polarity • Dipolar molecule
- 6. Covalent Bonding (electron sharing)
- 7. Polarity • Water molecule has a bent geometry: – Causes: • Overall (-) charge to side of O atom • Overall (+) charge to side of H atom • The separation of the charges gives the molecule an electrical polarity • Specifically, the water molecule is dipolar – Other common dipolar objects: • Flashlight batteries, car batteries, bar magnets
- 8. Dipolar molecule di = two, polus = pole • Water molecules behave like they have a tiny bar magnet inside • Weak negative charge at O end • Weak positive charge at H end • Hydrogen bonds • Weak bonds between water molecules and ions • Explains unusual properties of water
- 9. Two unusual properties 1. High surface tension – Hydrogen bonding creates “skin” – Important for living organisms • Capillarity 1. Universal solvent – Electrostatic bond between dipolar water and ions – Ocean is salty
- 10. Hydrogen Bond
- 11. Hydrogen Bonds Hydrogen Bond: between water molecules is much weaker than the covalent bonds that hold individual water molecules together. • Even though weaker than covalent bond still strong enough to show cohesion. – Cohesion causes water to bead up on a waxed surface • Cohesion also gives water its surface tension.
- 12. Surface Tension Water Piling Up) • Results from formation of hydrogen bonds between outermost layer of water molecules and underlying molecules (allowing water to “pile up”) • Capillarity: causes water Capillarity to climb up the side of a container due to the attraction between the (+) charge of water molecules and the (– )charge of the surface of the glass
- 13. Water: The Universal Solvent • Water sticks not only to other water molecules but also to other polar chemical compounds this means that water molecules can reduce the attraction between ions of opposite charges by as much as 80 times. • The reduced attraction allows water to dissolve nearly everything (everything polar that is). • Na+Cl- is dissolved very easily so the oceans are salty.
- 14. Na+ & Cl- ions Dissolved in Water Fig. 6-5b
- 15. Thermal properties of water • Solid, liquid, gas on Earth’s surface • Water has high freezing point • Water has high boiling point • Water has high heat capacity • Water has high latent heats
- 16. Thermal Properties Water’s Thermal Properties: • Influence world’s heat budget • Moderate coastal temperatures • In part responsible for development of tropical cyclones, worldwide wind belts, and ocean surface currents
- 17. Changing States of Matter • Add or remove Heat • Heat: is the energy of moving molecules. It is proportional to the energy level of molecules and therefore, is the total kinetic energy of a substance • Calorie: the amount of heat required to raise the temperature of 1 gram of water (~10 drops) by 1 degree centigrade. • Temperature: direct measure of the average kinetic energy of the molecules that make up a substance.
- 19. Heat Capacity • Amount of heat required to raise the temperature of 1 gram of any substance by 1 degree centigrade. • Water has the highest heat capacity and is exactly 1 calorie per gram (other substances are lower) • High Heat Capacity absorb (or lose) great amounts of heat with only a small change in temperature. • Low Heat Capacity substances that change temperature rapidly when heat is applied or taken away.
- 20. Water’s Latent Heats • Water undergoes changes of state. • During the changes: large amounts of heat is either absorbed or released because of water’s high latent (latent = hidden) heats. • These latent heats are closely related to water’s unusually high heat capacity. • Examples: – as water evaporates from your skin, it cools your body by absorbing heat (this is why sweating cools your body) – Opposite extreme: being scalded by water vapor/steam releases the latent heat onto your skin and gives you a severe burn when it condenses on your skin.
- 21. Fig. 6-7
- 24. Global thermostatic effects • Moderate global temperature • Evaporation removes heat from oceans • Condensation adds heat to atmosphere • Heat re-distributed globally
- 25. Differences in day and night temperatures
- 26. Water density • Maximum density at 4oC • Ice less dense than liquid water – Atomic structure of ice – Ice floats • Increased salinity decreases temperature of maximum density
- 29. Why Does Ice Float? • Density of most substances increases as it temperature decreases (ex: cold air sinks, warm air rises). • Density increases as temperature decreases because the molecules lose energy and slow down (so, same number of molecules occupy less space) thermal contraction • This also occurs in water but only to a certain point (to ~ 4oC (39oF). • From 4oC down to 0oC, its density decreases water stops contracting and actually expands causing it to be less dense than liquid water.
- 30. Seawater • Contains dissolved substances that give it a salty taste. • Dissolved substances are not strictly sodium and chloride but various salts, metals, and dissolved gases. Salinity: • The total amount of solid material dissolved in water (including gases some become solids at low enough temperatures)
- 31. Salinity of Seawater • 3.5% (35 0/00 ppt (parts per thousand)) • ~220 times saltier than fresh water. • Seawater with a salinity of 3.5% indicates that it also contains 96.5% pure water. • Physical properties are similar to those of pure water.
- 32. Parts Per Thousand (ppt) • A unit of measurement used in reporting salinity of water equal to the grams of dissolved substances in 1000 grams of seawater. • 10/00 is one part in 1000. • When converting from percent to ppt, the decimal is moved over one place to the right (ex: 3.5% = 350/00) • Advantages of Expressing Salinity in ppt: – Decimals are avoided and values convert to grams of salt per kilogram of seawater (ex: 35 0/00 seawater has 35 g of salt in 1000 g of seawater.
- 35. Salinity Variations • Open Ocean: varies between 33 and 38 o/oo • Brackish: (brak = salt, ish = somewhat) – Produced in areas where fresh water and seawater mix – Average salinity is ~ 10 o/oo (ex: Baltic Sea) • Hypersaline: (hyper = excessive, salinus = salt) – Typical of seas and inland bodies of water that experience high evaporation rates and limited open-ocean circulation – Salinities are well above 35 o/oo – Examples: Red Sea 42 o/oo, Great Salt Lake in Utah 280 o/oo, The Dead Sea 330 o/oo (10 times saltier than seawater) • Salinity of seawater also varies seasonally on coastal areas. Evaporation rates change throughout the year affecting salinity values.
- 36. Measuring Salinity • Evaporation – Evaporate a weighed amount then weighed salts that precipitated from it. • Chemical analysis – Principle of Constant Proportions • Major constituents of ocean-water salinity found in same relative proportions throughout the ocean-water volume, independent of salinity – Chlorinity • Amount of chloride ion(s) of other halogens in ocean water expressed in ppt(0/00) by weight. • Salinometer – Instrument that uses electrical conductivity to determine salinity of ocean water
- 38. Why Salinity Varies: Dissolved substances • Added to oceans – River input (primarily) – Circulation through mid-ocean ridges • Removed from oceans – Salt spray – Recycling through mid-ocean ridges – Biogenic sediments (hard parts and fecal pellets) – Evaporites
- 40. Residence time • Average length of time a substance remains dissolved in seawater • Long residence time = unreactive – Higher concentration in seawater • Short residence time = reactive – Smaller concentration in seawater • Steady state – Ocean salinity nearly constant through time
- 42. Dissolved gases • Solubility depends on temperature, pressure, and ability of gas to escape • Gases diffuse from atmosphere to ocean – Wave agitation increases amount of gas – Cooler seawater holds more gas – Deeper seawater holds more gas
- 43. Conservative vs. nonconservative constituents • Conservative constituents change slowly through time – Major ions in seawater • Nonconservative constituents change quickly due to biological and chemical processes – Gases in seawater
- 44. Oxygen and carbon dioxide in seawater • Nonconservative • O2 high in surface ocean due to photosynthesis • O2 low below photic zone because of decomposition • O2 high in deep ocean because source is polar (very cold) ocean
- 45. • CO2 low in surface ocean due to photosynthesis • CO2 higher below photic zone because of decomposition • Deeper seawater high CO2 due to source region and decomposition
- 46. Acidity and alkalinity • Acid releases H+ (hydrogen ion) when dissolved in water • Alkaline (or base) releases OH- (hydroxide ion) • pH scale measures acidity/alkalinity – Low pH value, acid – High pH value, alkaline (basic) – pH 7 = neutral
- 48. Carbonate buffering • Keeps ocean pH about same (8.1) • pH too high, carbonic acid releases H+ • pH too low, bicarbonate combines with H+ • Precipitation/dissolution of calcium carbonate CaCO3 buffers ocean pH • Oceans can absorb CO2 from atmosphere without much change in pH
- 49. Fig. 6-17
- 50. How salinity changes • Salinity changes by adding or removing water • Salinity decreases by – Precipitation (rain/snow) – River runoff – Melting snow
- 51. • Salinity increases by – Evaporation – Formation of sea ice • Hydrologic cycle describes recycling of water
- 54. Horizontal variations of salinity • Polar regions: salinity is lower, lots of rain/snow and runoff • Mid-latitudes: salinity is high, high rate of evaporation • Equator: salinity is lower, lots of rain • Thus, salinity at surface varies primarily with latitude
- 55. Fig. 6-20
- 56. Vertical variations of salinity • Surface ocean salinity is variable • Deeper ocean salinity is nearly the same (polar source regions for deeper ocean water) • Halocline, rapid change of salinity with depth
- 59. Density of seawater • 1.022 to 1.030 g/cm3 • Ocean layered according to density • Density of seawater controlled by temperature, salinity, and pressure – Most important influence is temperature – Density increases with decreasing temperature
- 60. • Salinity greatest influence on density in polar oceans • Pycnocline, rapid change of density with depth • Thermocline, rapid change of temperature with depth • Polar ocean is isothermal • Plimsoll Line, loading mark painted on the hull of merchant ships, it shows the depth to which a vessel may be safely (and legally) loaded.
- 64. Layers of ocean • Mixed surface layer • Pycnocline • Deep ocean
- 65. Major Gases in Atmosphere & Ocean