Thermal Energy
•Descargar como PPT, PDF•
5 recomendaciones•1,639 vistas
Introduction to thermal energy
Denunciar
Compartir
Denunciar
Compartir
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
Similar a Thermal Energy
Similar a Thermal Energy (20)
heat capacity and specific heat capacitylecture.pptx

heat capacity and specific heat capacitylecture.pptx
Último
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
This presentation was provided by William Mattingly of the Smithsonian Institution, during the third segment of the NISO training series "AI & Prompt Design." Session Three: Beginning Conversations, was held on April 18, 2024.Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"National Information Standards Organization (NISO)
Último (20)
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
IGNOU MSCCFT and PGDCFT Exam Question Pattern: MCFT003 Counselling and Family...

IGNOU MSCCFT and PGDCFT Exam Question Pattern: MCFT003 Counselling and Family...
Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...

Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...
Interactive Powerpoint_How to Master effective communication

Interactive Powerpoint_How to Master effective communication
Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"
social pharmacy d-pharm 1st year by Pragati K. Mahajan

social pharmacy d-pharm 1st year by Pragati K. Mahajan
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
BAG TECHNIQUE Bag technique-a tool making use of public health bag through wh...

BAG TECHNIQUE Bag technique-a tool making use of public health bag through wh...
Thermal Energy
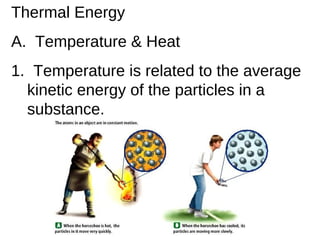
- 1. Thermal Energy A. Temperature & Heat 1. Temperature is related to the average kinetic energy of the particles in a substance.
- 2. 2. SI unit for temp. is the Kelvin a. K = C + 273 (10C = 283K) b. C = K – 273 (10K = -263C) 3. Thermal Energy – the total of all the kinetic and potential energy of all the particles in a substance.
- 3. 4. Thermal energy relationships a. As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). b. Even if the temperature doesn’t change, the thermal energy in a more massive substance is higher (because it is a total measure of energy).
- 4. 5. Heat a. The flow of thermal energy from one object to another. b. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler Cup gets cooler while hand gets warmer
- 5. 6. Specific Heat a. Some things heat up or cool down faster than others. Land heats up and cools down faster than water
- 6. b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C This is why land heats up quickly during the day and cools quickly at night and why water takes longer.
- 7. Why does water have such a high specific heat? Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them. water metal
- 8. How to calculate changes in thermal energy Q = m x T x C p Q = change in thermal energy m = mass of substance T = change in temperature (T f – T i ) C p = specific heat of substance
- 9. c. A calorimeter is used to help measure the specific heat of a substance. First, mass and temperature of water are measured Then heated sample is put inside and heat flows into water T is measured for water to help get its heat gain This gives the heat lost by the substance Knowing its Q value, its mass, and its T, its C p can be calculated