IB Chemistry on VSEPR
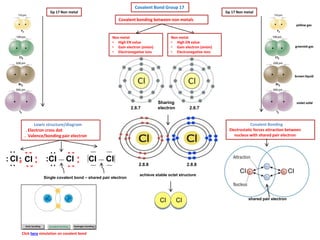
- 1. brown liquid Click here simulation on covalent bond yellow gas greenish gas violet solid Covalent bonding between non metals 2.8.7 Gp 17 Non metal achieve stable octet structure CI shared pair electron Covalent Bonding Electrostatic forces attraction between nucleus with shared pair electron 2.8.8 2.8.7 Sharing electron Gp 17 Non metal 2.8.8 CI Non metal • High EN value • Gain electron (anion) • Electronegative ions Covalent Bond Group 17 CICI Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: :. x x X x x x x CI CI :: x x x x CI CI Non metal • High EN value • Gain electron (anion) • Electronegative ions Single covalent bond – shared pair electron
- 2. Covalent bonding between non metals Covalent Bonding Single covalent bond ONE pair shared e 2.8.8 2.8.8 Covalent Bond Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: : : . x x X X x x x x x x O CI CI:: x x x x x x CI CI Sharing electron Bond Strength 2.8 2.8 Sharing electron O O: x x O N O O N: Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron C : :. N N: N N : Covalent Bonding Double covalent bond TWO pair shared e Covalent Bonding Triple covalent bond THREE pair shared e O x x: CO CO O O O Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Covalent Bonding Double covalent bond TWO pair shared e
- 3. : . CI .. x Bond Bond order Bond strength Bond length/pm C - C 1 347 154 C = C 2 612 134 C Ξ C 3 820 120 N - N 1 159 145 N = N 2 418 123 N Ξ N 3 914 110 Bond length and Bond strength Bond length = 0.199nm Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: : : . x x X X x x x x O CI CI:: x x x x x x CI CI O O: x x O N O O N: Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron O : : . N N: N N : Triple bond > Double bond > Single bond Bonding pair e -involve in bonding Bond length decrease Bond strength Increase (Double/Triple bond) Bond length = 0.121nm Bond length = 0.110nm Bond order up – Bond strength up – Bond length down O: Non bonding pair (Lone pair electron) Bonding pair electron C O: : Bonding pair electron Dative bond (electron pair of oxy) Types of bonding Lone pair e –not involve in bonding Dative/coordinate bond - pair e come from an atom Exception to octet rule All period 2 element - observe octet rule except Be and B Electron deficient Less than 8 valence e Expanded octet More than 8 valence e All period 3 element - observe octet rule except P and S : BeCI CIx . : :: :: x x. Be - 4 valence e BCI CI : :: : :: x : :B - 6 valence e P S CI CI CI CI CI CI CICI CICI CI P - 10 valence e S – 12 valence e
- 4. Valence Shell Electron Pair Repulsion Predict molecular shape/geometry Shape determine by electron pairs/ electron charge centers/ECC Bonding/lone pair – repel each other Bonding/lone pair arrange themselves as far as possible (minimise repulsion) Valence Shell Electron Pair Repulsion NOT surrounding atoms N H HH .. Principles of VSEPR Shape of molecule Determine number valence e around central atom1 2 Single, double, triple bond , lone pair act as electron charge center/ECC 3 4 Lone pair-lone pair > Lone pair-bonding pair > bonding pair- bonding pair repulsion 5 6 ECC or electron pair position in equatorial first, then axial Excellent VSEPR simulation Click here ✓ Click here VSEPR notes Lewis structure VSEPR .. N H H H Shape Click here VSEPR simulation
- 5. Valence Shell Electron Pair Repulsion Bonding/lone pair arrange themselves as far as possible (minimise repulsion) Principles of VSEPR Determine number valence e around central atom1 2 Single, double, triple bond , lone pair act as electron charge center/ECC 3 Bonding/lone pair repel each other Lone /lone pair > Lone /bond pair > bond/bond pair repulsion 4 5 For 5/6 ECC: ECC position in equatorial first, then axial .. N HHH 3 bonding pair 1 lone pair 4 ECC N – central atom 3 ECC C H =O H H C N 2 ECC OH H 4 ECC > > 1 lone pair2 lone pair 0 lone pair Repulsion greater - Bond angle smaller Repulsion greater Repulsion greater ✓ ECC far apart – Bond angle greatest – minimise repulsion 6 Lone pair need more space Multiple bonds more space Unequal repulsionEqual repulsion 90° 120° 109.5° 107° 180°
- 6. H Bond angle - 104.5° Linear Bond angle - 180° O OO C BeH N B X x C SO O C H NO C OO CH N HH Be N OX x X X X X X X X x X x + + : F F F H H O O O C O O O B X F F F C H H x x O S O O C O x x O 2- 2- O x x : || BF3 CH2 O SO3 CO3 2- CO2 HCN BeH2 NO2 +Lone Pair Bonding Pair Geometry 2 bond pair ✓ = = Geometry 3 bond pair Bond angle - 120° Trigonal planar ✓ E L E C T R O N C H A R G E C E N T E R O3 O O O : O O : O NO2 N OO N OO NO2 - N OO : N OO : SO2 OO - - S : S OO : Geometry 2 bond pair 1 lone pair Bent ✓ E L E C T R O N C E N T E R
- 7. Bond angle -109.5° Bond angle -104.5° O Bond angle- 107° Tetrahedral Trigonal Pyrimidal N H N : : O SO3 2-PH3NH3 O HH H2O H H O O F2O F F F F O S CICI SCI2 N H H S CI CI N H NH2 - - - H: : : : : : : : : : : Bent CIO3 - H H P H H H H H H P H H H : O O S O S O : O 2- 2- CI O O O CI : O O - : CH4 C H NH4 + BH4 - PCI4 + H H H H C H H H N H H H H H N H H H H B HH H H B H H H CI CI CI CI P CI CI CI P CI - - + + + + :: : :: :: : : :: : : : : : Lone Pair Bonding Pair E L E C T R O N C H A R G E C E N T E R Geometry 2 bond pair 2 lone pair Geometry 3 bond pair 1 lone pair ✓ Geometry 4 bond pair ✓ - ✓
- 8. : : : : : : Linear Bond angle 180° Bond angle <90° T shape Bond angle <90° , <120° Trigonal Bipyrimidal Bond angle - 90° , 120° P CI CI CI CI CI : P CI CI CI CI S F F F F S F F F F Te CI CI CI CI Te CI CI CI CI : CI F F F CI F F F I CI CI CI CI I CI CI I F F F F F I F F F Xe F F O O Xe F F O O : + + : CI I I I Xe CI CI I F F ::::::: : : :: :: :: : : : : : :: : : :: : : : : : :: :: : :::: : : :: : :: : :: :: : :: : :: : :: : :: :: :: :: :: :: : BrF F F Br F F F : : : : : : :: : - PCI5 SF4 TeCI4 (IF4)+ XeO2F2 CIF3 ICI3 BrF3 (I3)- (ICI2)- XeF2 - Bonding Pair Lone Pair E L E C T R O N C H A R G E C E N T E R : : : : Geometry 5 bond pair ✓ Geometry 4 bond pair 1 lone pair Seesaw ✓ Geometry 3 bond pair 2 lone pair ✓ 2 bond pair 3 lone pair (XeF3 )+ F F Xe F F Xe F F :: : :: :: :: + + ✓ Geometry
- 9. Linear Square planar F S SF6 F F F F F PCI6 - P CI CI CI CI CI CI IF5O I O || F F F F F F S F F F F F CI P CI CI CI CI CI I F F F F F :: O Square pyrimidal CI Sb Sb CI CI CICI CI CI CICI CI (SbCI5)2- BrF5 Xe F FF F Xe XeF4 F F F Br F FF F F Br F F O F F Xe || F FF F O :: Xe F F F F F Te F FF F F Te F F F F XeOF4 (TeF5)- - - CICI I CICI (ICI4)- - : : : : : : :: : : : :: ::: : :: : :::: :: : : : : : : : :: : : : : :: : ::: : : : : :: : :: : :::: : :::: :::: : : : :: :: : : : : : :::: :::: : : ::: :::: : :: : : : : : : :: ::: :::: : 2- 2- : - - Lone Pair Bonding Pair :: :: : :::: :::: : : : : : : : : : : :: :: : (XeF3) - F (XeF2)2- F F Xe E L E C T R O N C H A R G E C E N T E R Lone pair in equatorial first, then axial Lone pair in equatorial first, then axial : : : : - 2- Minimise repulsion Minimise repulsion Geometry 6 bond pair Bond angle - 90° Octahedral ✓ Geometry 5 bond pair 1 lone pair Bond angle < 90° ✓ 4 bond pair 2 lone pair Bond angle - 90° ✓ 3 bond pair 3 lone pair Bond angle - <90° T shape ✓ 2 bond pair 4 lone pair Bond angle - 180° ✓
- 10. H Bond angle 104.5° Linear Bond angle 180° O OO C BeH N B X x C SO O C H NO C OO CH N HH Be N OX x X X X X X X X x X x + + : F F F H H O O O C O O O B X F F F C H H x x O S O O C O x x O 2- 2- O x x : || BF3 CH2 O SO3 CO3 2- CO2 HCN BeH2 NO2 +Lone Pair Bonding Pair ECC = 2 2 bond pair ✓ = = ECC = 3 3 bond pair Bond angle 120° Trigonal planar ✓ E L E C T R O N C H A R G E C E N T E R O3 O O O : O O : O NO2 N OO N OO NO2 - N OO : N OO : SO2 OO - - S : S OO : ECC = 3 2 bond pair 1 lone pair Bent ✓ E L E C T R O N C E N T E R Equal repulsion Electron Distribution (LINEAR) Equal repulsion Electron Distribution (TRIGONAL PLANAR) Unequal repulsion Electron Distribution (TRIGONAL PLANAR)
- 11. Bond angle 109.5° Bond angle 104.5° O Bond angle 107° Tetrahedral Trigonal Pyrimidal N H N : : O SO3 2-PH3NH3 O HH H2O H H O O F2O F F F F O S CICI SCI2 N H H S CI CI N H NH2 - - - H: : : : : : : : : : : Bent CIO3 - H H P H H H H H H P H H H : O O S O S O : O 2- 2- CI O O O CI : O O - : CH4 C H NH4 + BH4 - PCI4 + H H H H C H H H N H H H H H N H H H H B HH H H B H H H CI CI CI CI P CI CI CI P CI - - + + + + :: : :: :: : : :: : : : : : Lone Pair Bonding Pair E L E C T R O N C H A R G E C E N T E R 2 bond pair 2 lone pair ECC = 4 3 bond pair 1 lone pair ✓ ECC = 4 4 bond pair ✓ - ✓ ECC = 4 Electron Distribution (TETRAHEDRAL) Unequal repulsion Unequal repulsion Electron Distribution (TETRAHEDRAL) Equal repulsion Electron Distribution (TETRAHEDRAL)
- 12. Bond angle 180° Linear Bond angle <90° T shape Bond angle < 90° , < 120° Trigonal BipyrimidalBond angle 90° , 120° P CI CI CI CI CI : P CI CI CI CI S F F F F S F F F F Te CI CI CI CI Te CI CI CI CI : CI F F F CI F F F I CI CI CI CI I CI CI I F F F F F I F F F Xe F F O O Xe F F O O : + + : CI I I I Xe CI CI I F F ::::::: : : :: :: :: : : : : : :: : : :: : : : : : :: :: : :::: : : :: : :: : :: :: : :: : :: : :: :: :: :: :: :: :: :: :: : : : : BrF F F Br F F F : : : : : : :: : - PCI5 SF4 TeCI4 (IF4)+ XeO2F2 CIF3 ICI3 BrF3 (I3)- (ICI2)- XeF2 - Bonding Pair Lone Pair E L E C T R O N C H A R G E C E N T E R : : : : ECC = 5 5 bond pair ✓ ECC = 5 4 bond pair 1 lone pair Seesaw ✓ ECC = 5 3 bond pair 2 lone pair ✓ ECC = 5 2 bond pair 3 lone pair F F Xe F F Xe F F :: : :: :: :: + + ✓ Equal repulsion Electron Distribution (TRIGONAL BIPYRIMIDAL) Unequal repulsion Electron Distribution (TRIGONAL BIPYRIMIDAL) Unequal repulsion Electron Distribution (TRIGONAL BIPYRIMIDAL) Electron Distribution (TRIGONAL BIPYRIMIDAL)
- 13. Linear Square planar F S SF6 F F F F F PCI6 - P CI CI CI CI CI CI IF5O I O || F F F F F F S F F F F F CI P CI CI CI CI CI I F F F F F :: O Square pyrimidal CI Sb Sb CI CI CICI CI CI CICI CI (SbCI5)2- BrF5 Xe F FF F Xe XeF4 F F F Br F FF F F Br F F O F F Xe || F FF F O :: Xe F F F F F Te F FF F F Te F F F F XeOF4 (TeF5)- - - CICI I CICI (ICI4)- - : : : : : : :: : : : :: ::: : :: : :::: :: : : : : : : : :: : : : : :: : ::: : : : : :: : :: : :::: : :::: :::: : : : :: :: : : : : : :::: :::: : : ::: :::: : :: : : : : : : :: ::: :::: : 2- 2- : - - Lone Pair Bonding Pair :: :: : :::: :::: : : : : : : : : : : :: :: : (XeF3) - F (XeF2)2- F F Xe E L E C T R O N C H A R G E C E N T E R Lone pair in equatorial first, then axial Lone pair in equatorial first, then axial : : : : - 2- Minimise repulsion Minimise repulsion ECC = 6 6 bond pair Bond angle 90° Octahedral ✓ ECC = 6 5 bond pair 1 lone pair Bond angle < 90° ✓ 4 bond pair 2 lone pair ✓ 3 bond pair 3 lone pair Bond angle < 90° T shape ✓ 2 bond pair 4 lone pair Bond angle 180° ✓ Equal repulsion Electron Distribution (OCTAHEDRAL) Unequal repulsion Electron Distribution (OCTAHEDRAL) Bond angle 90° Electron Distribution (OCTAHEDRAL) Electron Distribution (OCTAHEDRAL) Unequal repulsion Electron Distribution (OCTAHEDRAL)
- 14. Valence Shell Electron Pair Repulsion Predict molecular shape/geometry Shape determine by electron pairs/ electron charge centers/ECC Bonding/lone pair – repel each other Bonding/lone pair arrange themselves as far as possible (minimise repulsion) Valence Shell Electron Pair Repulsion N H HH .. Principles of VSEPR Shape of molecule Determine number valence e around central atom1 2 Single, double, triple bond , lone pair act as electron charge center/ECC 3 4 Lone pair-lone pair > Lone pair-bonding pair > bonding pair- bonding pair repulsion 5 6 ECC or electron pair position in equatorial first, then axial Lewis structure VSEPR .. N H H H Geometry 4 ECC 3 bonding pair 1 lone pair Trigonal pyrimidal 1 2 3 Bond pair electron • Occupy smaller region space bet nuclei • Repulsion less Lone pair electron nucleus > Bonding pair electron Concept Map nuclei Lone pair electron • Electron pair occupy greater space • Repel any bonding pair nearby • Lone pair repulsion > bonding pair repulsion Double bond •Repulsion greater •Angle smaller, 111.4° B F F F Single bond •Equal repulsion •Angle 120° 120° 120° 120° space occupy by electron space occupy by electron
