Determination of reaction order.
•
6 recomendaciones•6,907 vistas
Determination of reaction order. Physical Pharmaceutics - 2 Soniya.M.Sunil 3rd year B.Pharm Mar Dioscorus College of Pharmacy.
Denunciar
Compartir
Denunciar
Compartir
Descargar para leer sin conexión
Recomendados
Recomendados
Más contenido relacionado
La actualidad más candente
La actualidad más candente (20)
Unit iii heterocyclic compounds as per PCI Syllabus of POC-III

Unit iii heterocyclic compounds as per PCI Syllabus of POC-III
Unit V: Reaction of synthetic importance as per PCI Syllabus of POC-III

Unit V: Reaction of synthetic importance as per PCI Syllabus of POC-III
Unit i.Optical Isomerism as per PCI syllabus of POC-III 

Unit i.Optical Isomerism as per PCI syllabus of POC-III
Suspension, type of suspension, interracial property of suspended particles 

Suspension, type of suspension, interracial property of suspended particles
Similar a Determination of reaction order.
Similar a Determination of reaction order. (20)
Chemical kinetics_Rate laws and reaction mechanisms.pdf

Chemical kinetics_Rate laws and reaction mechanisms.pdf
Último
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Último (20)
Food Chain and Food Web (Ecosystem) EVS, B. Pharmacy 1st Year, Sem-II

Food Chain and Food Web (Ecosystem) EVS, B. Pharmacy 1st Year, Sem-II
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
Asian American Pacific Islander Month DDSD 2024.pptx

Asian American Pacific Islander Month DDSD 2024.pptx
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources

Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources
Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes

Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes
Role Of Transgenic Animal In Target Validation-1.pptx

Role Of Transgenic Animal In Target Validation-1.pptx
Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...

Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Determination of reaction order.
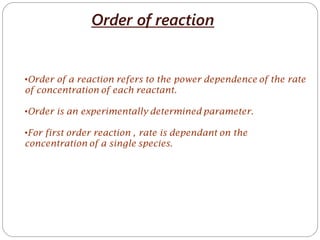
- 1. Order of reaction •Order of a reaction refers to the power dependence of the rate of concentration of each reactant. •Order is an experimentally determined parameter. •For first order reaction , rate is dependant on the concentration of a single species.
- 2. Determination of reaction order ❖Exact order of a reaction can be determined experimentally. ❖The methods to determine reaction order are: •Graphical Method •Substitution Method •Half life Method •Van’t Hoff differential Method •Ostwald’s isolation Method
- 3. Graphical Method ▪In presence of single reactant. ▪Rate versus concentration graph: •‘Log(a-x)’ vs ‘t’ : straight line = first order reaction. •‘1/(a-x)’ vs ‘t’ : straight line = second order reaction. •‘1/(a-x)^2’ vs ‘t’ : straight line = third order reaction.
- 4. Substitution Method •Hit and trial method. •Integrated rate law method. •Concentration and time will be given. •The value of k is determined . •If the value of k is constant ,the used equation gives the order of reaction.
- 5. Half-life method • Only used when the rate law involve one concentration term. • t(1/2) ∞ a1-n t(1/2) = k’a1-n log t(1/2) = log k’ + (1-n)loga • Graph can be drawn where the slope is (1-n) , n = order of reaction. • If different concentrations are given the ratio of half life is taken. , • By taking logs and rearranging,
- 6. Van’t Hoff differential method •The rate of a reaction varies as the ‘n’th power of the concentration of the reactant , where, n= reaction order. • Thus, for two different initial concentrations C1 and C2, equations can be written in the form: , • Taking logarithm and subtracting, •Or , n = [log(-(dC1)/dt)-log((dC2)/dt)] ÷ [logC1 - log C2] ❑ nA Product rate = k[A] ⁿ dxldt = k[A] ⁿ log(dxldt) = logk + nlogA y = c + mx (Slope = n)
- 7. Ostwald’s isolation method • Initial rate method. • Initial rate of reaction is determined by varying the concentration of the reactants while others are kept constant. • For a reaction given by: aA + bB → cC + dD • Rate ∝ [A]x[B]y ⇒ Rate = k[A]x[B]y , where , • [A] and [B] are concentration of reactants . • k is the constant. • x and y are the partial reaction order. • If [B] is kept constant, • R(1) = k[A1]x • R(2) = k[A2]x • R(1) / R(2) = ( [A1]/[A2])x
- 8. Thank You.. Soniya . M . Sunil B. Pharm 3rd year Mar Dioscorus College of Pharmacy