Gc Chemical Kinetics
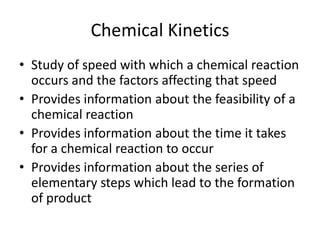
- 1. Chemical Kinetics Study of speed with which a chemical reaction occurs and the factors affecting that speed Provides information about the feasibility of a chemical reaction Provides information about the time it takes for a chemical reaction to occur Provides information about the series of elementary steps which lead to the formation of product
- 2. Rate Data for A + B -> C
- 3. A + B -> C C A B
- 4. The Rate of a Chemical Reaction The speed of a reaction can be examined by the decrease in reactants or the increase in products. a A + b B -> c C + d D Where m and n are determined experimentally, and not necessarily Equal to the stiochiometry of the reaction
- 5. Reaction A -> 2 B A A B B B B A B B B B A A A B B B B A A A B B B B A B B B B 22 22 = 6.022 x 10 molecules B 6.022 x 10 molecules = A in a 1.00 L container in a 1.00 Liter container 1 mol/L 2 mol/L
- 6. Average Rate Rate of A disappearing is Let’s suppose that after 20 seconds ½ half of A disappears. Then And Rate of B appearing is Then
- 7. Average Rate Law for the General Equationa A + b B -> c C + d D For Example: N2O5 (g) -> 2 NO2 (g) + ½ O2 (g)
- 8. Determination of the Rate Equation Determined Experimentally Can be obtained by examining the initial rate after about 1% or 2% of the limiting reagent has been consumed.
- 9. Consider the Reaction:CH3CH2CH2CH2Cl (aq) + H2O (l) -> CH3CH2CH2CH2OH (aq) + HCl (aq)
- 10. Average Rates,
- 11. Average Rates,
- 12. Average Rates,
- 13. Average Rates,
- 14. Instantaneous Rate or initial rate at t=0 s Instantaneous Rate at t = 500 s
- 16. Order of Reaction Zero order –independent of the concentration of the reactants, e.g, depends on light First order - depends on a step in the mechanism that is unimolecular Pseudo first order reaction – one of the reactants in the rate determining step is the solvent Second order – depends on a step in the mechanism that is bimolecular Rarely third order – depends on the step in the mechanism that is termolecular
- 17. Data from the hydrolysis of n-butyl chloride
- 18. IF Zero Order
- 19. Therefore, the reaction is not zero order
- 20. If Second Order
- 21. Therefore, the reaction is not second order
- 22. IF First Order Reaction
- 23. First Order Plot
- 24. First Order Plot
- 25. Slope
- 26. Slope
- 27. Rate of the Reaction
- 28. For the ReactionN2O5 (g) -> 2 NO2 (g) + ½ O2 (g) The rate can be used to explain the mechanism
- 29. (1) Slow Step . (2) Fast Step .
- 30. Sum of the two steps: N2O5 -> 2 NO2 + ½ O2 or 2 N2O5 -> 4 NO2 + O2
- 31. ApplicationMechanism of a Chemical Reaction (a) Suggest a possible mechanism for NO2 (g) + CO (g) -> NO (g) + CO2 (g) Given that (b) Suggest a possible mechanism for 2 NO2 (g) + F2 (g) -> 2 NO2F (g) Given that
- 32. Factors Affecting the Rate of a Chemical Reaction The Physical State of Matter The Concentration of the Reactants Temperature Catalyst
- 33. For A Reaction to Occur Molecules Must Collide Molecules must have the Appropriate Orientation Molecules must have sufficient energy to overcome the energy barrier to the reaction- Bonds must break and bonds must form
- 34. A Second Order Reaction
- 35. Rate Constant “k” Must be determined experimentally Its value allows one to find the reaction rate for a new set of concentrations
- 36. The following data were collected for the rate of the reaction Between A and B, A + B -> C , at 25oC. Determine the rate law for the reaction and calculate k.
- 37. From Experiments 1 and 2 Solution A: Divide equation (1) into equation (2)
- 38. Solution B: Subtract equation (2) from equation (1)
- 39. From Experiments 4 and 5 Solution A: Divide equation (1) into equation (2)
- 40. Solution B: Subtract equation (2) from equation (1)
- 41. Rate Constant k
- 42. Rate Constant kFrom Experiment 3
- 43. Rate Constant kFrom Experiment 1
- 44. Your Understanding of this ProcessConsider the Data for the Following Reaction: Determine the Rate Law Expression and the value of k consistent With these data.
- 45. From Experiments 1 and 2 Solution : Divide equation (1) into equation (2)
- 46. From Experiments 2 and 3 Solution : Divide equation (1) into equation (2)
- 47. Rate Expression
- 48. AssignmentDetermine the Rate Law for the following reaction from the given data: 2 NO (g) + O2 (g) -> 2 NO2 (g)
- 49. Relationship Between Concentration and Time First Order Reaction
- 50. A plot of Gives a Straight line The Following Reaction is a First Order Reaction: Plot the linear graph for concentration versus time and obtain the rate constant for the reaction.
- 51. Data for the Transformation of cylcpropane to propene
- 52. slope = 2 x 10-5 s-1
- 53. Data for the Transformation of cylcpropane to propene
- 54. -slope = -2 x 10-5s-1 Slope = 2 x 10-5 s-1
- 55. Relationship Between Concentration and Time Second Order Reaction
- 56. A plot of Gives a Straight line The Following Reaction is a Second Order Reaction: 2 HI (g) -> H2 (g) + I2 (g) Plot the linear graph for concentration versus time and obtain the rate constant for the reaction.
- 57. Data for the Transformation of hydrogen iodide gas to hydrogen and iodine
- 58. slope = 30. L mol-1 min-1
- 59. Graphical Method for Determining the Order of a Reaction First Order Reaction: y = ln (ao – x); x = t; slope = -k; and the intercept is lnao or y = ln (ao /(ao – x)); x = t; slope = k; and the intercept =0 Second Order Reaction: y = 1/ (ao – x); x = t; slope = k; and the intercept = 1/ ao Zero Order Reaction: y = x; x = t; slope = k and the intercept = 0 or y = ao – x ; x = t; slope = -k and the intercept = ao
- 61. Application of the Graphical Method for Determining the Order of a Reaction N2O5 (g) -> 2 NO2 (g) + ½ O2 (g) Tabulate the data so that each order may be tested
- 62. Data tabulation to determine which order will give a linear graph
- 63. Test for zero order reaction Not linear; therefore, the reaction is not zero order
- 64. Test for first order reaction Linear; therefore, the reaction is first order
- 65. Test for second order reaction Non-linear; therefore, the reaction is not second order
- 66. Half Life for a First Order Reaction
- 67. Half Life for a Second Order Reaction
- 68. Half Life for a Zero Order Reaction
- 69. Application of Half Life The rate constant for transforming cyclopropane into propene is 0.054 h-1 Calculate the half-life of cyclopropane. Calculate the fraction of cyclopropane remaining after 18.0 hours. Calculate the fraction of cyclopropane remaining after 51.5 hours.
- 70. Half-Life Fraction of cyclopropane Remaining after 18.0 hours Fraction of cyclopropane Remaining after 51.5 hours
- 71. Effect of Temperature on the Reaction Rate Arrhenius Equation
- 72. Use the Following Data to Determine the Eactfor
- 74. Effect of a Catalyst on the Rate of a Reaction Lowers the energy barrier to the reaction via lowering the energy of activation Homogeneous catalyst- in the same phase as the reacting molecules Herterogeneous catalyst – in a different phase from the reacting molecules
- 75. Example of a Homogeneous Catalyst
- 77. Example of Heterogeneous Catalyst
- 78. An interesting problem: The reaction between propionaldehyde and hydrocyanic acid have been observed by Svirbely and Roth and reported in the Journal of the American Chemical Society. Use this data to ascertain the order of the reaction and the value of the rate constant for this reaction.
- 80. Check to determine first order in HCN
- 81. Check to determine first order in propionaldehyde
- 82. So close; therefore, let’s take another approach. Let [HCN] = ao and [propionaldehyde] = bo Then,
- 85. Let’s construct the data in a different format
- 86. Slope = 0.678; therefore, k = 0.678 M-1min-1
- 87. Mechanism:
- 88. Steps 1 and 2 are fast equilibrium steps; and step 3 is the rate determining step
- 91. Revisit the kinetics for 2 NO (g) + O2 (g) -> 2 NO2 (g)