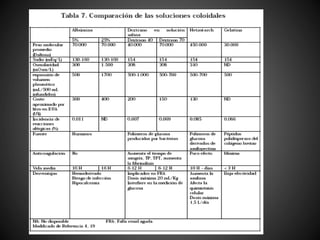
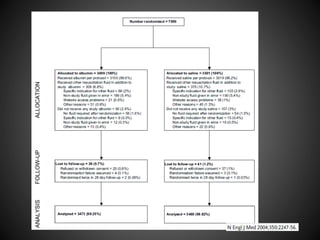
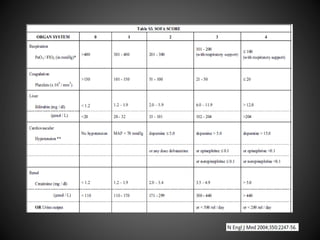
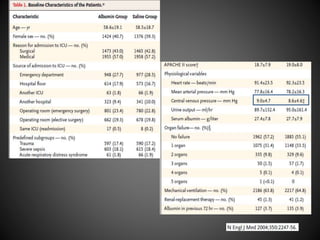
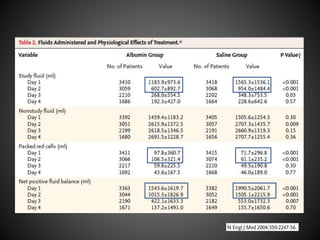
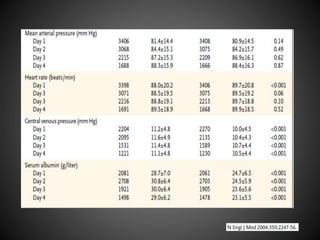
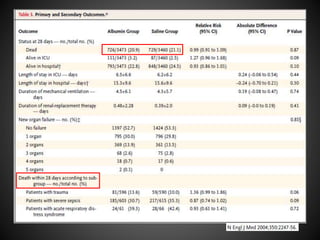
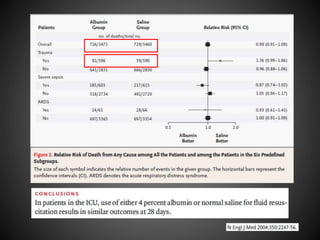
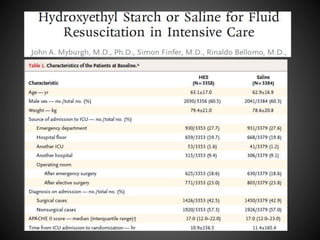
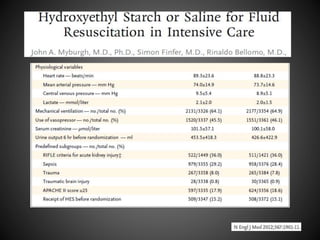
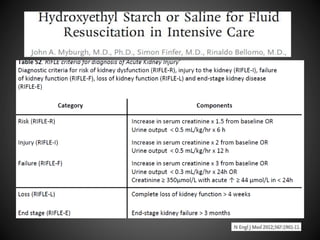
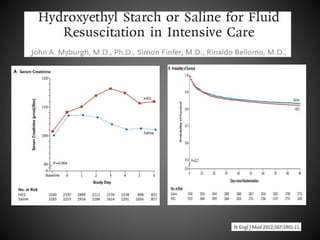
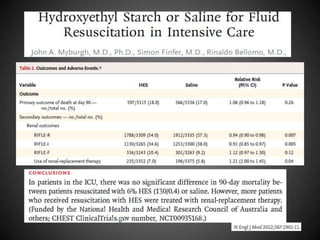
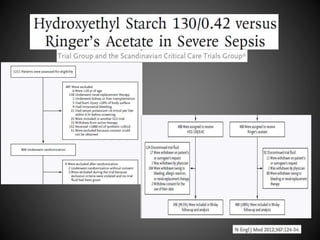
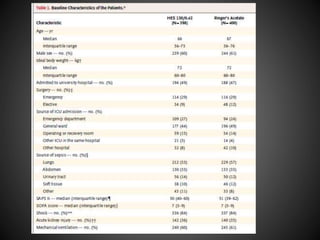
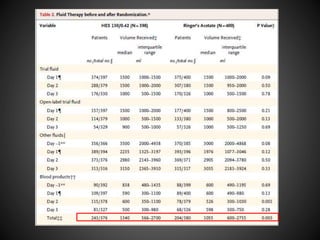
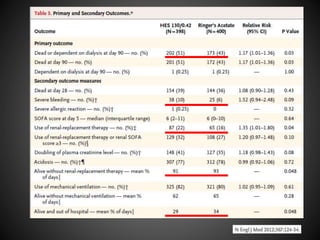
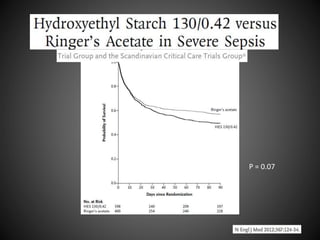
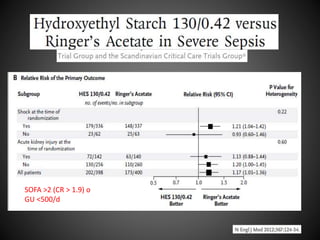
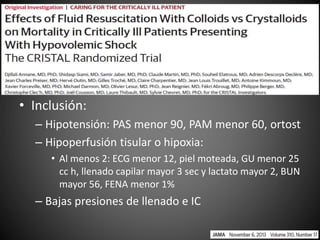
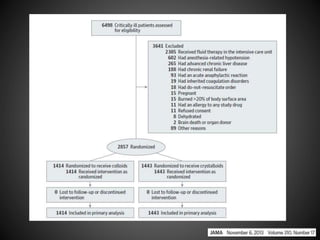
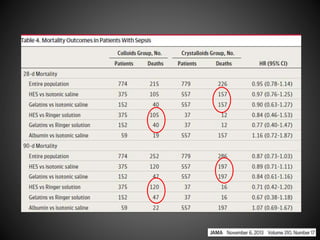
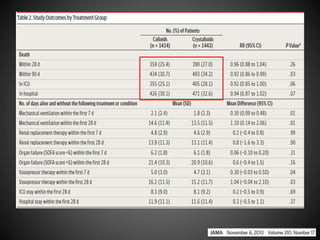
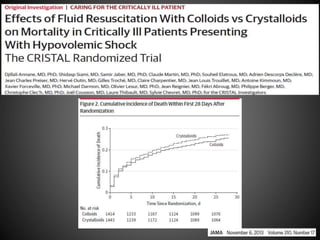
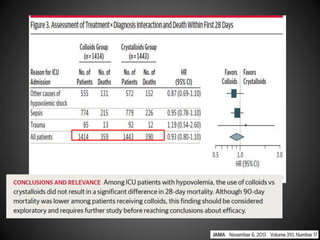
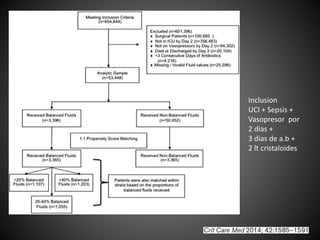
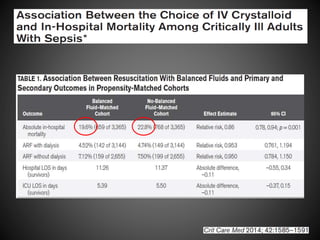
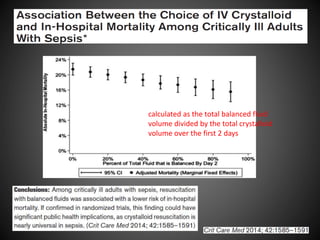
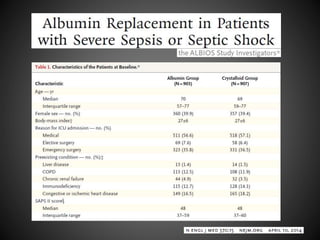
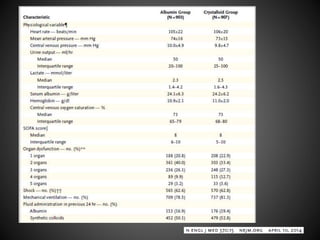
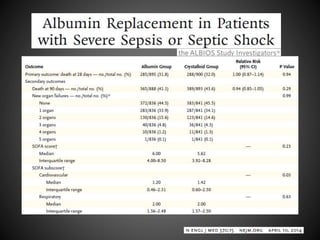
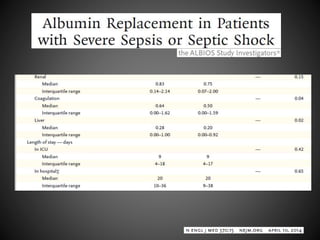
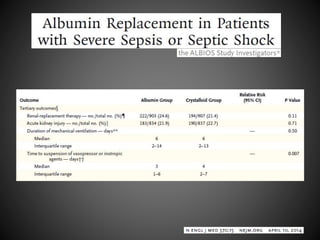
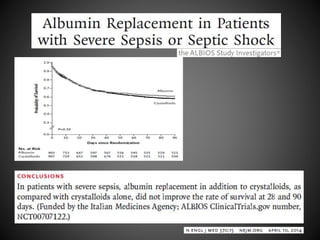
Este documento resume varios estudios clínicos aleatorizados que comparan el uso de coloides versus cristaloides para la reanimación de volumen en pacientes con sepsis. Los estudios no encontraron diferencias significativas en la mortalidad entre los grupos tratados con albúmina u otros coloides sintéticos en comparación con los tratados solo con sueros cristaloides. Sin embargo, los coloides sintéticos podrían estar asociados con mayores riesgos renales.